Original Paper
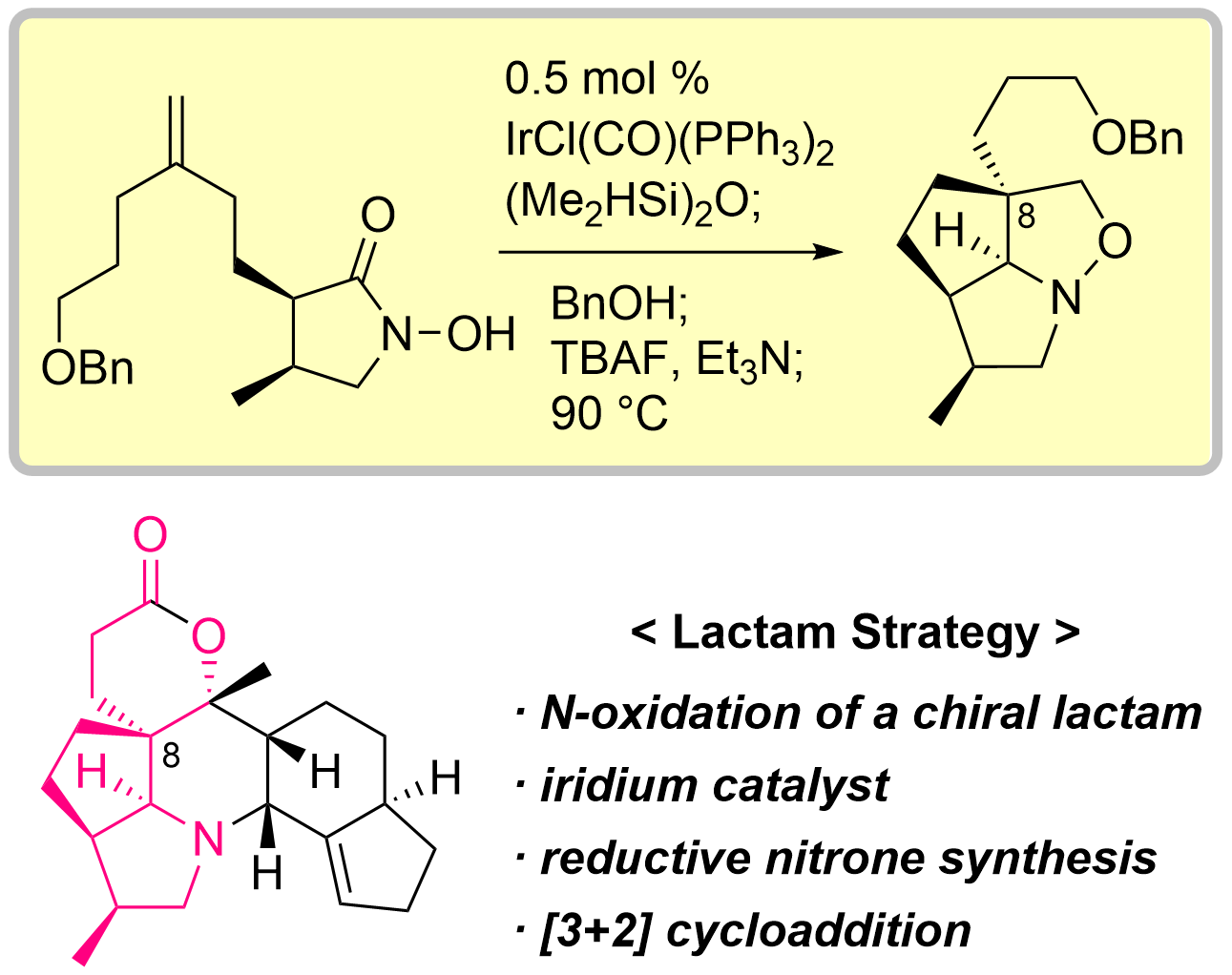
83. Total Synthesis of Isodaphlongamine H by Iridium-catalyzed Reductive [3+2] Cycloaddition of N-Hydroxylactam
Iwamoto, S.; Nakano, R.; Sasaki, K.; Kobayashi, S.; Taira, Y.; Takei, K.; Kawakita, R.; Tokuyama, A.; Nakamura, H.; Tomoike, M.; Kawahara, R.; Murase, A.; Simizu, S.; Chida, N.; Okamura, T.; *Sato T.
Angew. Chem. Int. Ed. 2025, 64, e08062.
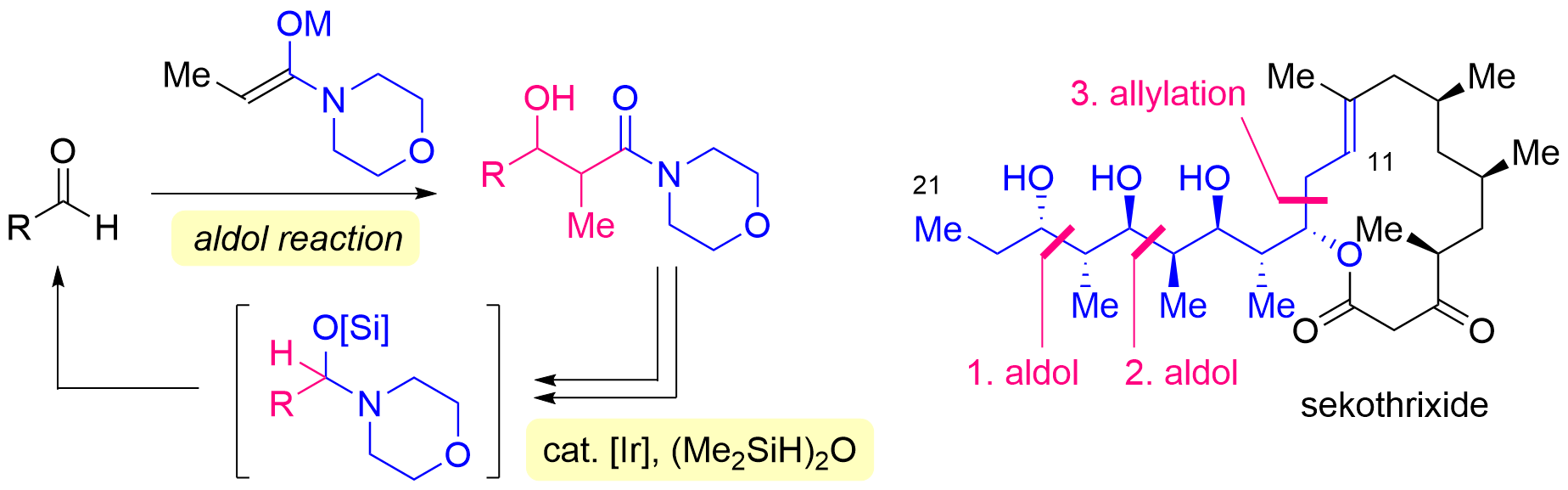
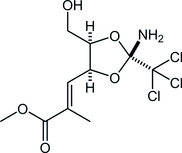
82. Synthesis of C11-C21 Polyol Fragment of Sekothrixide Based on an Iterative Approach Including Iridium-catalyzed Hydrosilylation of Morpholine Amides
Yasui, S.; Aoki, S.; Okamura, *Sato T.
Chem. Lett. 2025, 54, upaf094.
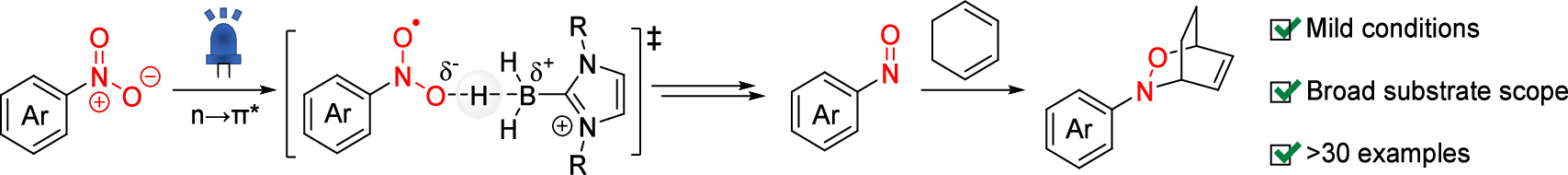
81. Photoinduced Reductive [4 + 2] Cycloaddition of Nitroarenes
Kaneko, T.; Ito R.; *Okamura, T.; *Sato, T.
Org. Lett. 2025, 27, 2042–2048.
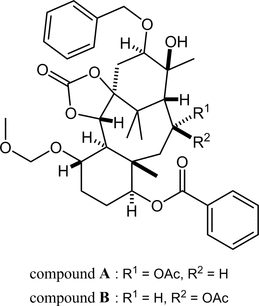
80. Crystal structures of (±)-(1SR,5SR,6SR,7SR,10SR,11SR,13RS,14SR,15SR,16RS)-13-acetoxy-16-benzyloxy-15-hydroxy-7-methoxymethoxy-3-oxo-11,15,18,18-tetramethyl-2,4-dioxatetracyclo[12.3.1.01,5.06,11]octadecan-10-yl benzoate and its 13-epimer
*Oishi, T.; Fukaya, K.; Sato, T.; Chida, N.
Acta Cryst. 2025, E81, 74–79.
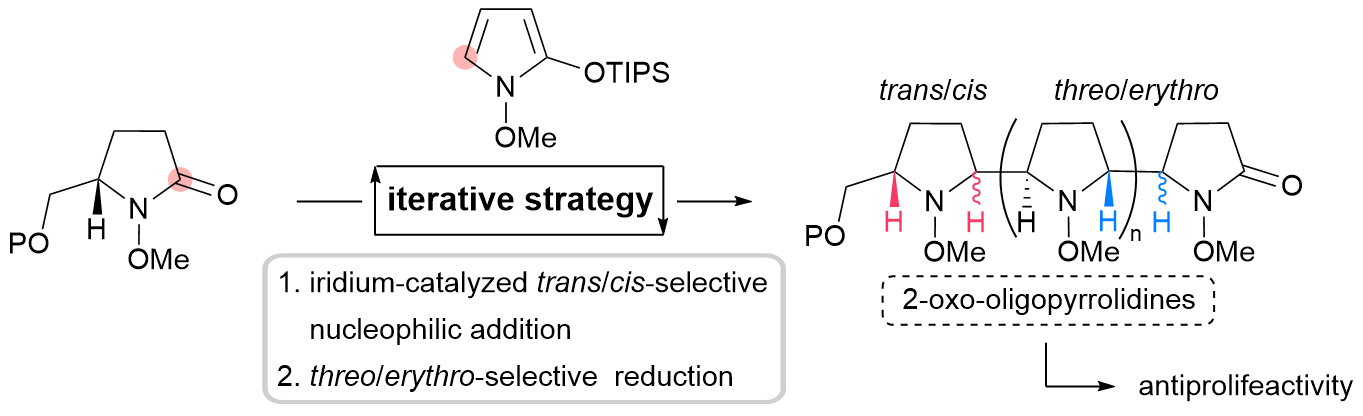
79. Stereodivergent Synthesis of 2-Oxo-oligopyrrolidines by Iterative Coupling Strategy
Soda, Y.; Tatsumi, K.; Forner, M.; Sato, S.; Shibuya, K.; Matagawa, T.; Simizu, S.; Chida, N.; *Okamura, T.; *Sato T.
Org. Biomol. Chem. 2024, 22, 3230−3236.
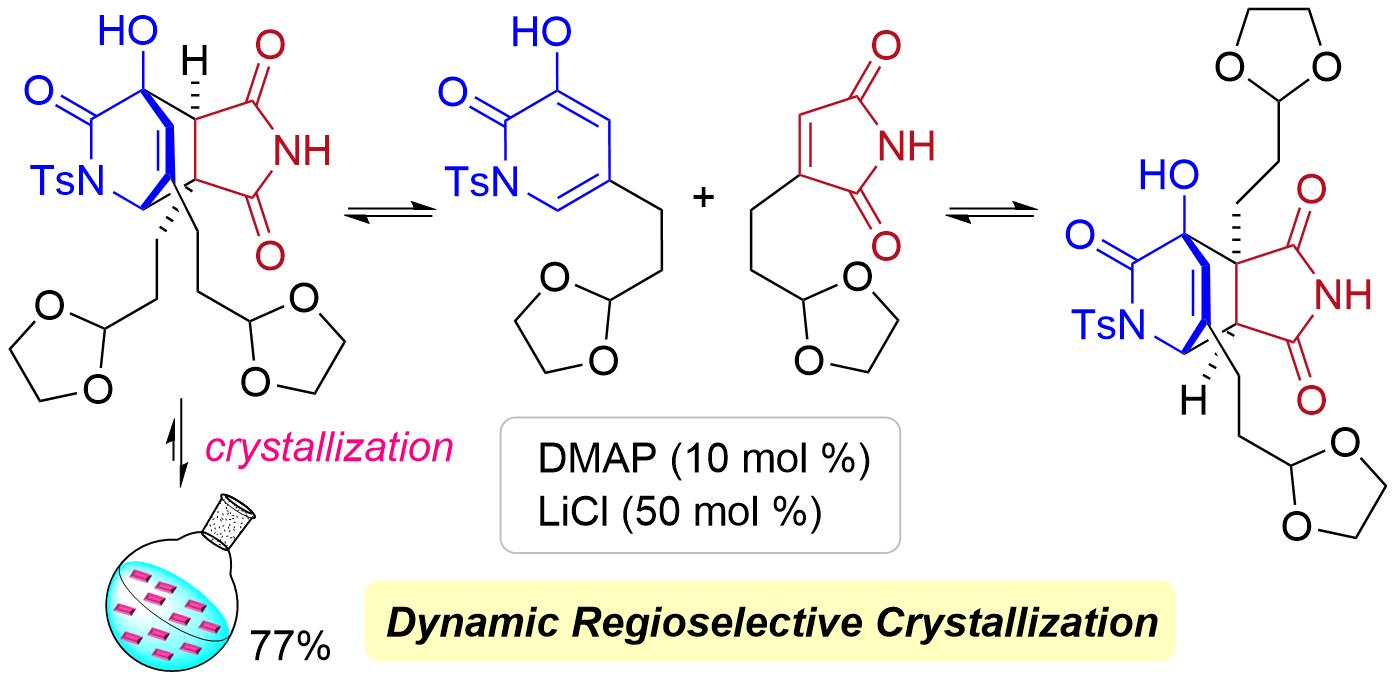
78. Total Synthesis of Keramaphidin B and Ingenamine by Base-Catalyzed Diels-Alder Reaction Using Dynamic Regioselective Crystallization
Kurihara, Y.; Yagi, M.; Noguchi, T.; Yasufuku, H.; Okita, A.; Yoshimura, S.; Oishi, T.; Chida, N.; Okamura, T.; *Sato T.
J. Am. Chem. Soc. 2024, 146, 11054–11060.
This article was selected as the most accessed articles on a monthly basis.
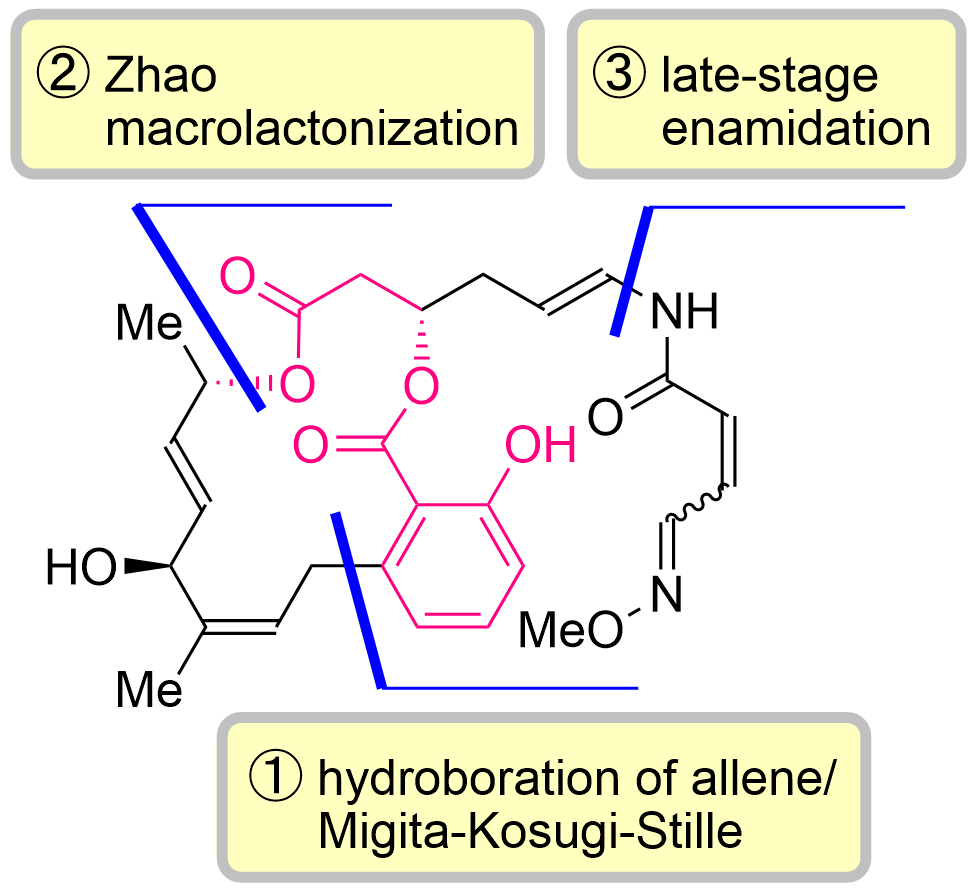
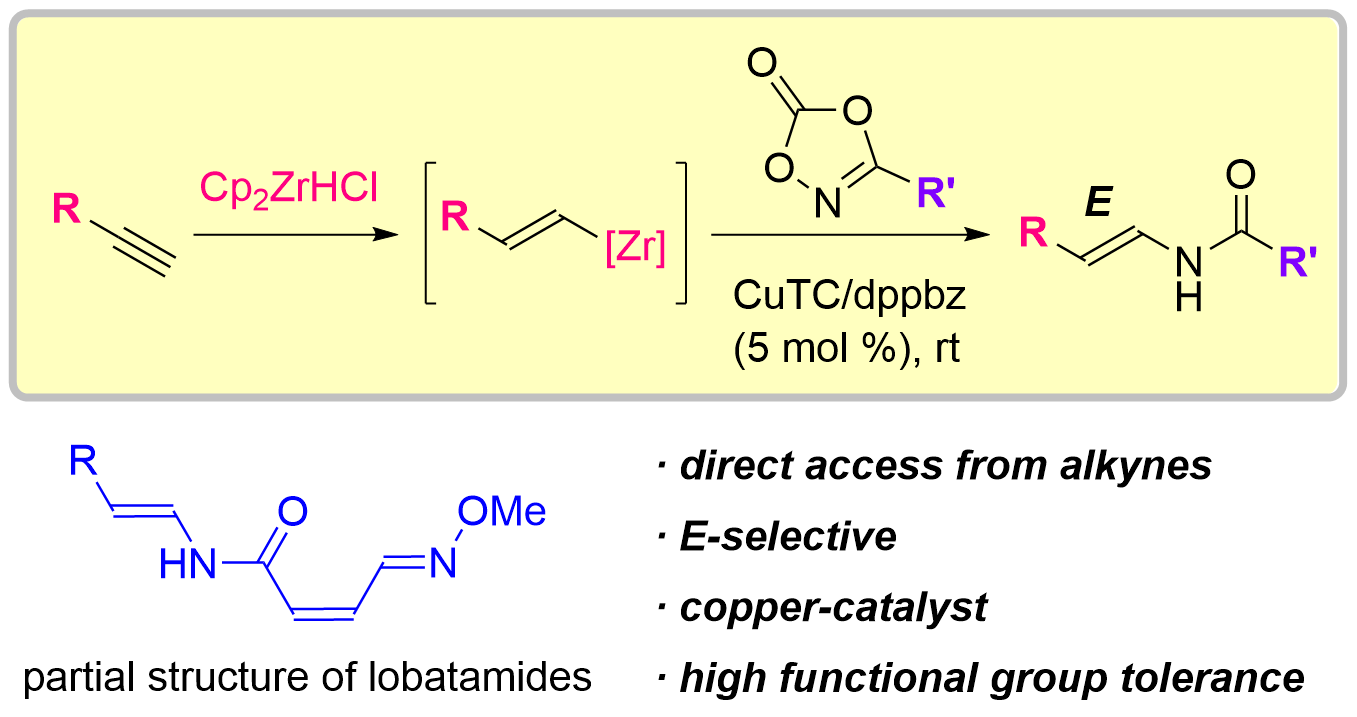
77. Total Synthesis of Lobatamides A and C
Yasui, S.; Banjo, S.; Nagashima, Y.; Okada, Y.; Yoshikawa, K.; Nakata, K.; Chida, N.; Okamura, T.; *Sato T.
Angew. Chem. Int. Ed. 2024, 63, e202402335.
This article was selected as the most accessed articles in 03/2024.
76. Synthesis and Evaluation of Saponins with All-Nitrogenated Sugars
Kage, A.; Okuyama, Y.; Kato, E.; Matagawa, T.; Kawano, S.; Simizu, S.; Chida, N.; *Okamura, T.; *Sato T.
Synlett 2024, 35, 427–430.
This article was invited as the cluster of 11th Singapore International Chemistry Conference (SICC-11)
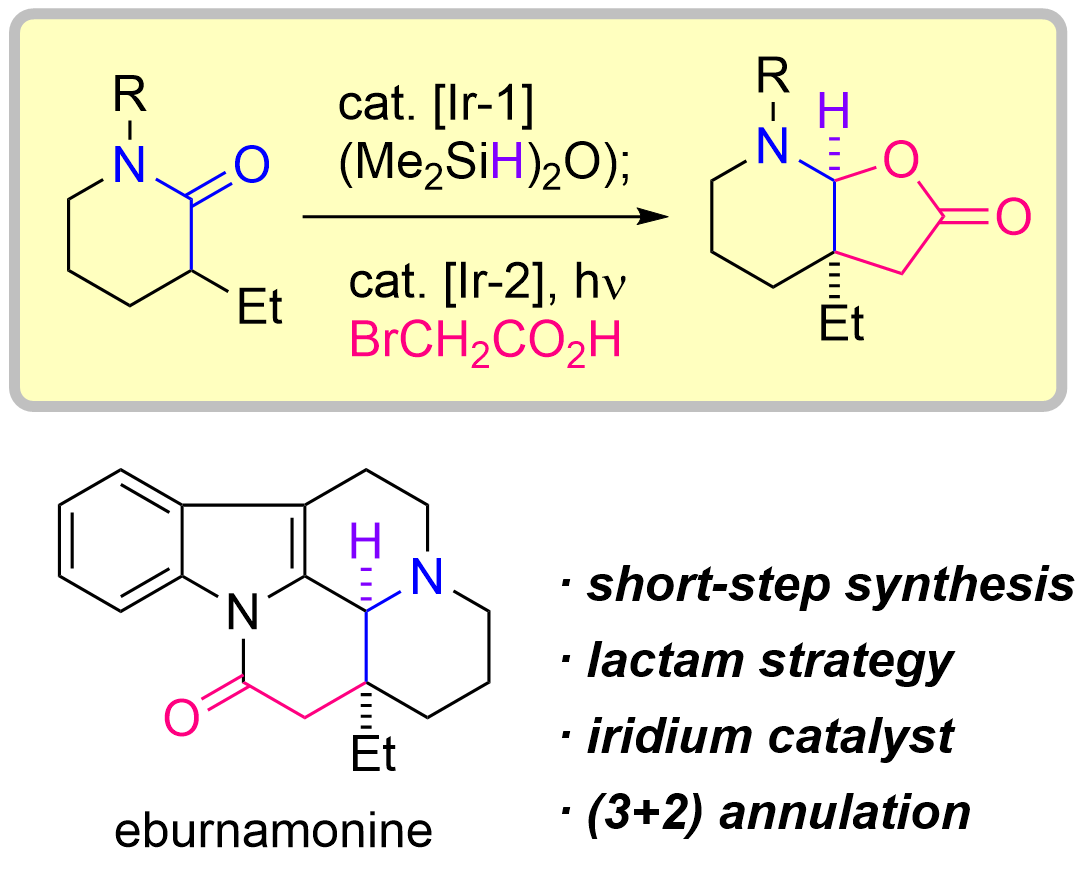
75. Iridium-Catalyzed Reductive (3+2) Annulation of Lactams Enabling the Rapid Total Synthesis of (±)-Eburnamonine
Sugiyama, Y.; Yamada, K.; Kaneko, D.; Kusagawa, K.; Okamura, T.; *Sato T.
Angew. Chem. Int. Ed. 2024, 63, e202317290.
74. Computational study focusing on a comprehensive conformational analysis of transition states for aza-spiro ring formations with N-alkoxyamides
*Fukaya, K.; Sato, T.; Chida, N.; Urabe, D.
J. Org. Chem. 2023, 88, 13655–13665.
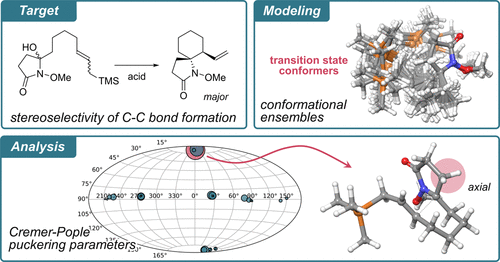
73. Iridium-catalyzed Reductive Olefination of N-Methoxyamides
Iiyama, S.; Mizutani, K.; *Sato, T.
Chem. Lett. 2023, 52, 682–684.
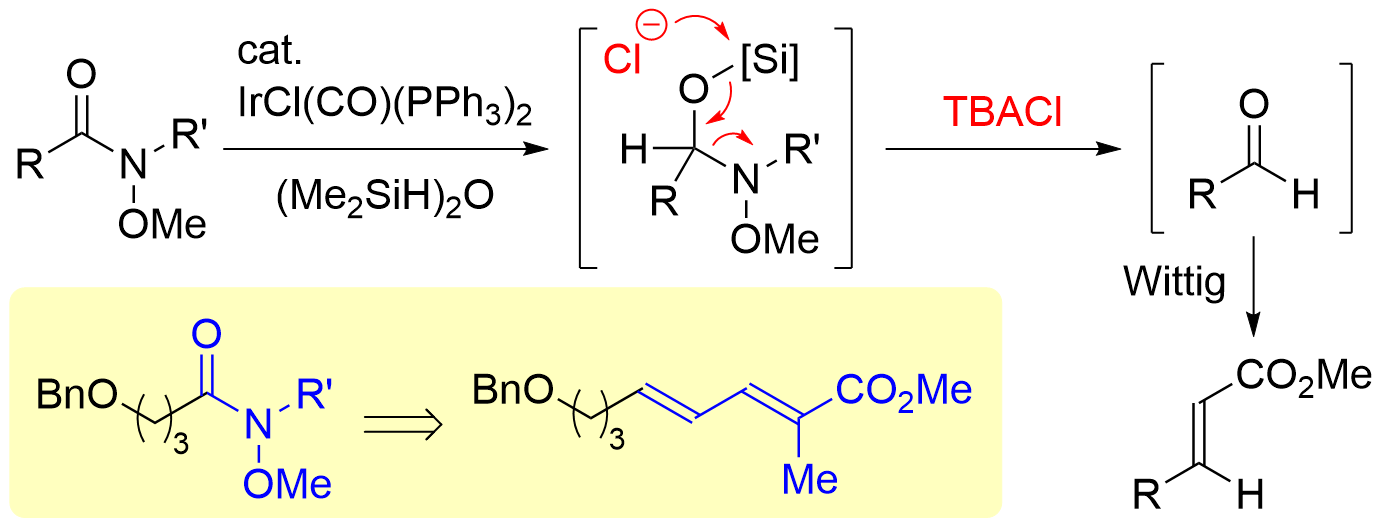
72. Synthesis of Chiral α,α-Disubstituted Cyclic Nitrones from Secondary Lactams
Iwamoto, S.; Tokuyama, A.; Hiraoka, S.; Takei, K.; Matsuzaka, K.; Matsumoto, T.; Chida N.; *Sato T.
Bull. Chem. Soc. Jpn. 2023, 96, 529–537.
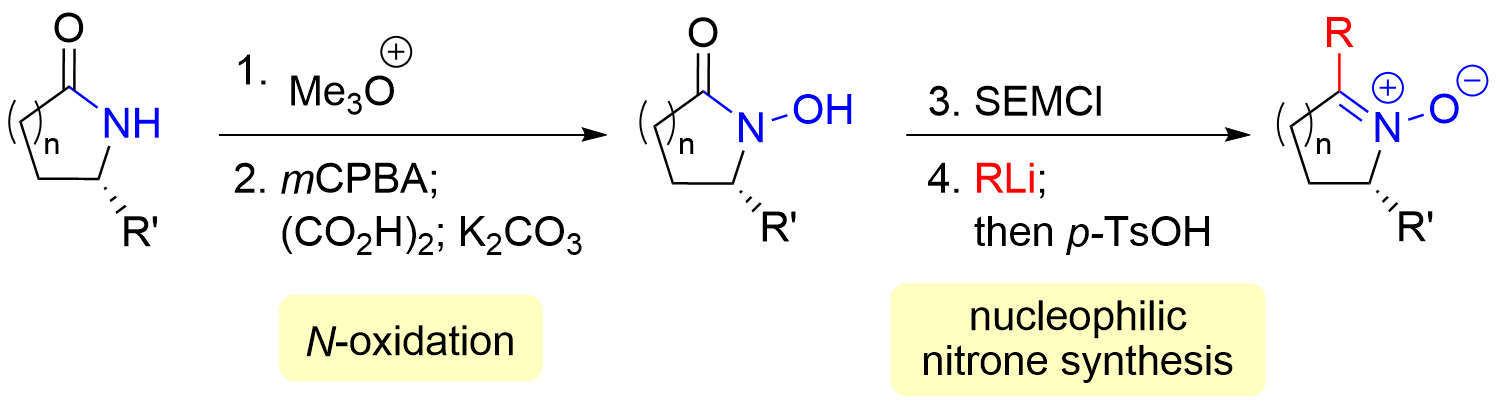
71. Total Synthesis and Anti-inflammatory Activity of Stemoamide-Type Alkaloids Including Totally Substituted Butenolides and Pyrroles
Soda, Y.; Sugiyama, Y.; Sato, S. Shibuya, K.; Saegusa, J.; Matagawa, T.; Kawano, S.; Yoritate, M.; Fukaya, K.; Urabe, D.; Oishi, T.; Mori, K.; Simizu, S.; Chida, N.; *Sato, T.
Synthesis 2023, 55, 617–636.
This paper was selected as the SYNTHESIS best paper award 2023.
This article was highlighted in Synform 2022/12, A202–A206.
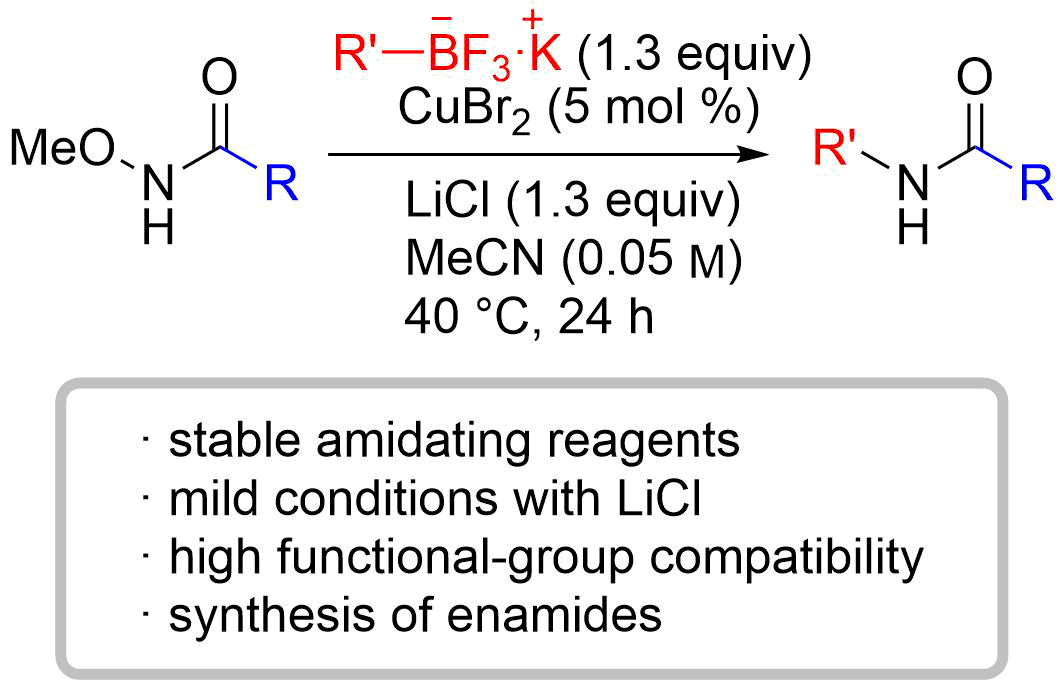
70. Copper-Catalyzed Electrophilic Enamidation Using Dioxazolones through Hydrozirconation of Alkynes
Banjo, S.; Nakata, K.; Nakasuji, E.; Yasui, S.; Chida, N.; *Sato, T.
Org. Lett. 2022, 24, 8662–8666.
69. Synthesis of Two Macrocyclic Fragments of Manadomanzamine Alkaloids
Kurihara, Y.; Azuma, A.; Yasufuku, H.; Takikawa, S.; Chida, N.; *Sato, T.
Chem. Lett. 2022, 51, 1146–1149.
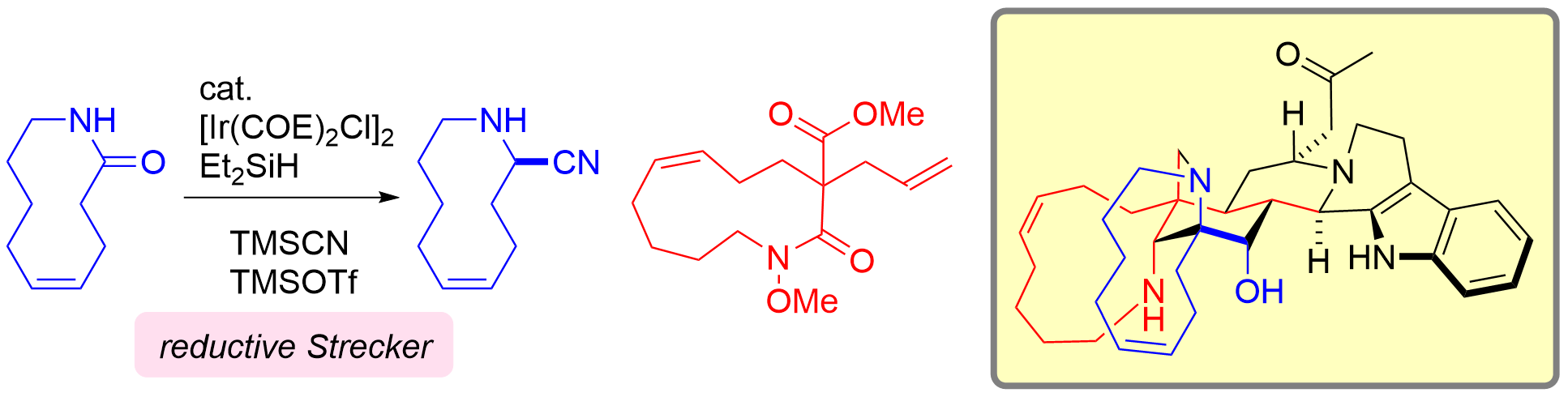
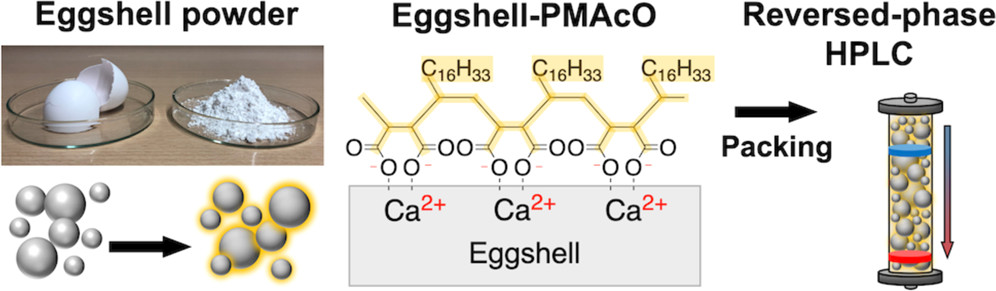
68. Amphiphilic Copolymer-Modified Eggshell-Based Column Packing 2 Materials for the Preparative Separation of Basic Drugs
Yoshii, T.; Mochida, M.; Kaizu, K.; Soda, Y.; Kanamori, K.; Nakanishi, K.; Sato, T.; Imai, H.; Citterio, D.; *Hiruta, Y.
ACS Appl. Polym. Mater. 2022, 4, 6949–6957.
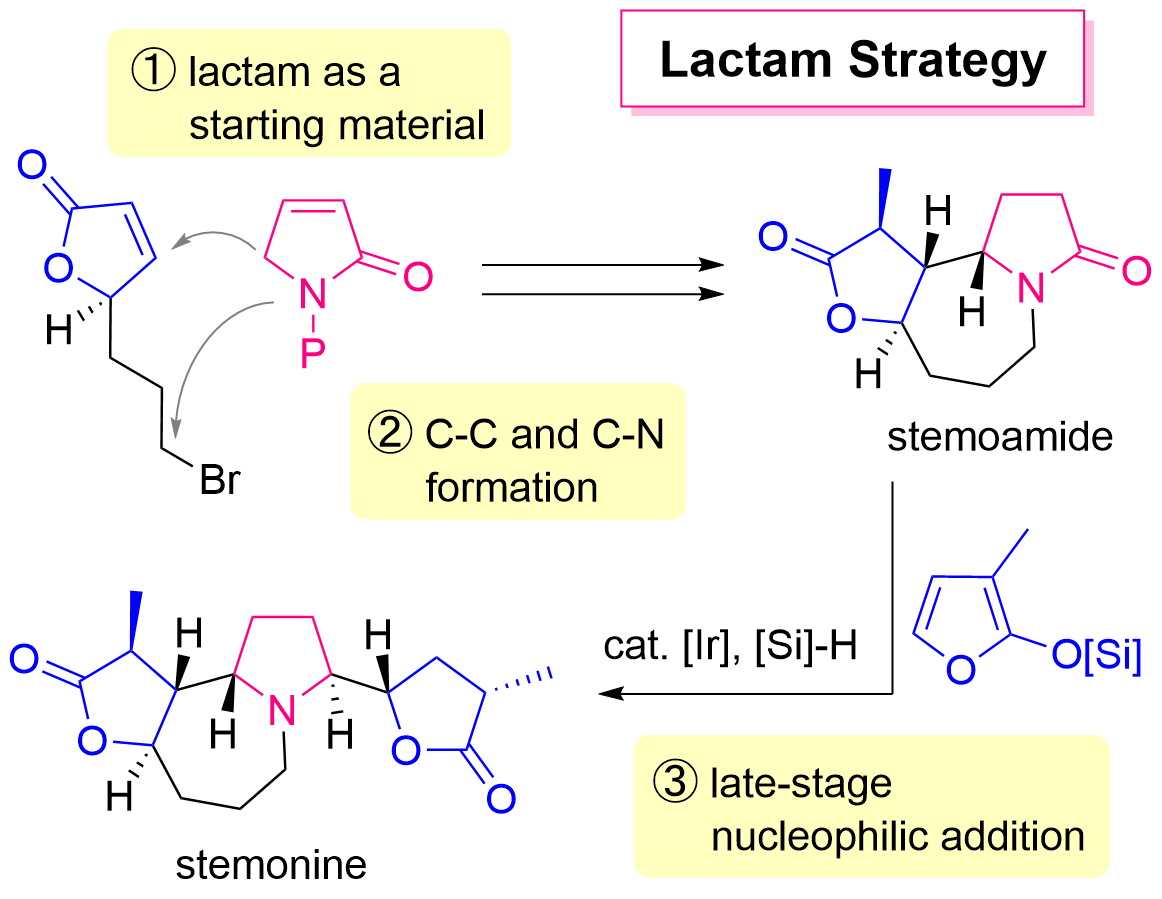
67. Lactam Strategy Using Amide-Selective Nucleophilic Addition for the Quick Access to Complex Amines: Unified Total Synthesis of Stemoamide-Type Alkaloids
Sugiyama, Y.; Soda, Y.; Yoritate, M.; Tajima, H.; Takahashi, Y.; Shibuya, K.; Ogihara, C.; Yokoyama, T.; Oishi, T.; *Sato, T.; *Chida, N.
Bull. Chem. Soc. Jpn. 2022, 95, 278–287.
This article was selected as the Selected Paper.
This article was selected as the inside cover.
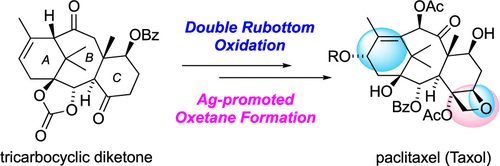
66. Total Synthesis of Paclitaxel
Iiyama, S.; Fukaya, K.; Yamaguchi, Y.; Watanabe, A.; Yamamoto, H.; Mochizuki, S.; Saio, R.; Noguchi, T.; Oishi, T.; Sato, T.; *Chida, N.
Org. Lett. 2022, 24, 202–206.
This article was selected as the most read articles on a monthly basis.
This article was selected as the most read articles on an annual basis.
65. Crystal structure of (+)-(1S,5S,6S,7S,10S,11S,16S)-16-hydroxy-7-(methoxymethoxy)- 11,15,18,18-tetramethyl-3,13-dioxo-2,4-dioxatetracyclo[12.3.1.01,5.06,11]octadec-14-en-10-yl benzoate
*Oishi, T.; Fukaya, K.; Sato, T.; Chida, N.
Acta Cryst. 2021, E77, 1234–1238.
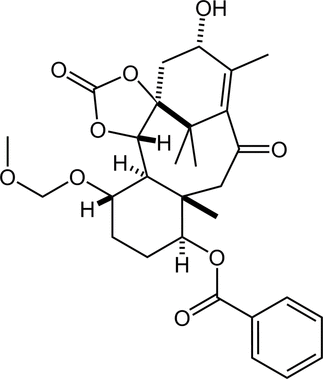
64. Development of Chiral N-Alkoxyamide Strategy and Application to Asymmetric Total Synthesis of Fasicularin
Minamikawa, R.; Fukaya, K.; Kobayashi, A.; Komiya, Y.; Yamamoto, S.; Urabe, D.; Chida, N.; *Sato, T.
Synthesis 2021, 53, 4621–4635.
This Feature Article was invited.
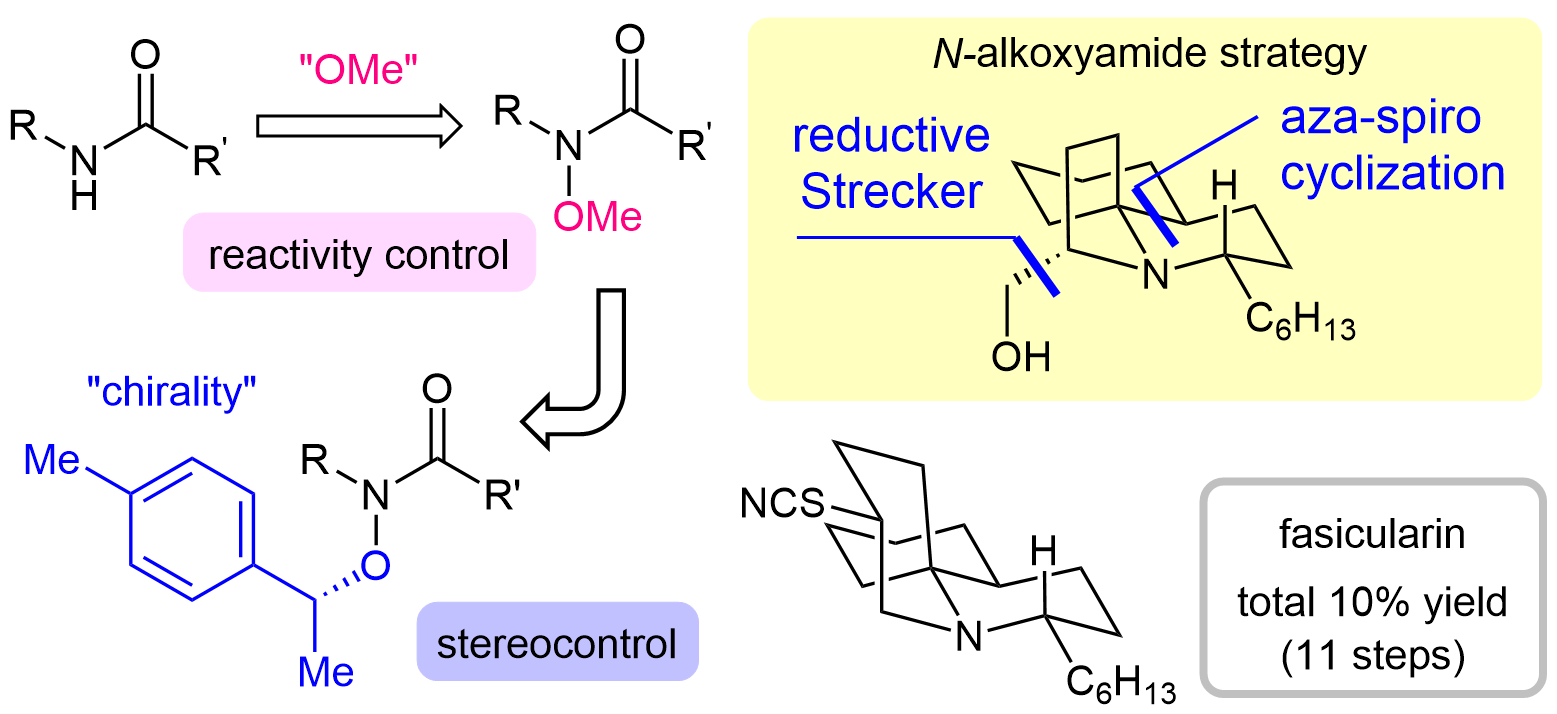
63. Five-Step Total Synthesis of (±)-Aspidospermidine by a Lactam Strategy via an Azomethine Ylide
Katahara, S.; Sugiyama, Y.; Yamane, M.; Komiya, Y.; *Sato, T.; *Chida, N.
Org. Lett. 2021, 23, 3058–3063.
This article was selected as the most read articles on a monthly basis.
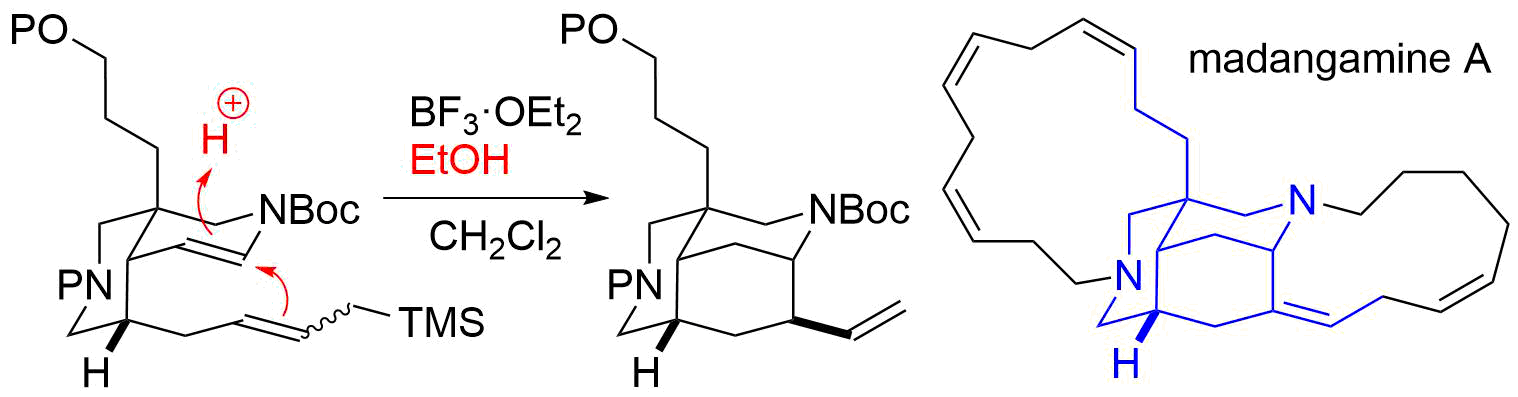
62. Identification of madangamine A as a novel lysosomotropic agent to inhibit autophagy
Miura, K.; Kawano, S.; Suto, T.; Sato, T.; Chida, N.; *Simizu S.
Bioorg. Med. Chem. 2021, 34, 116041.
This article was selected as a front cover.

61. Seven-step Synthesis of All-nitrogenated Sugar Derivatives Using Sequential Overman Rearrangements
Okuyama, Y.; Kidena, M.; Kato, E.; Kawano, S.; Ishii, K.; Maie, K.; Miura, K.; Simizu, S.; *Sato, T.; *Chida, N.
Angew. Chem. Int. Ed. 2021, 60, 5193–5198.
This article was selected as a Hot Paper.
This article was selected as an inside back cover.

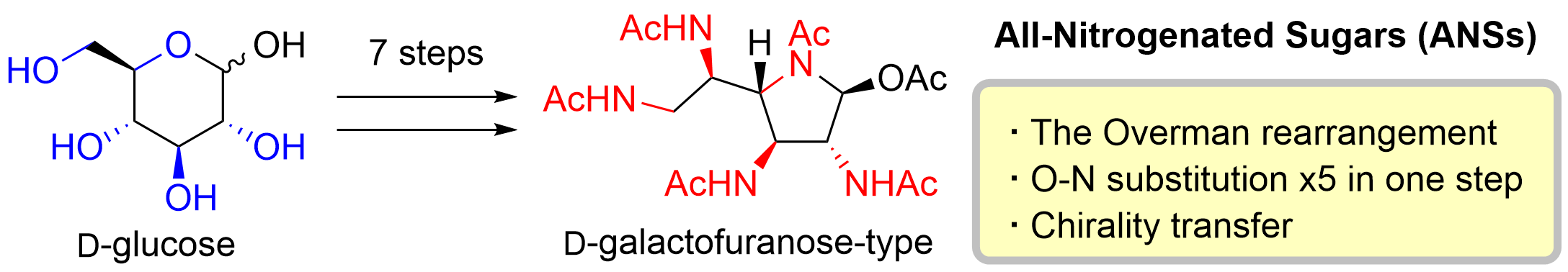
60. Synthesis of Saxitoxin and Its Derivatives
Okuyama, Y.; Okamoto, R.; Mukai, Kinoshita, K.; Sato, T.; *Chida, N.
Org. Lett. 2020, 22, 8697–8701.
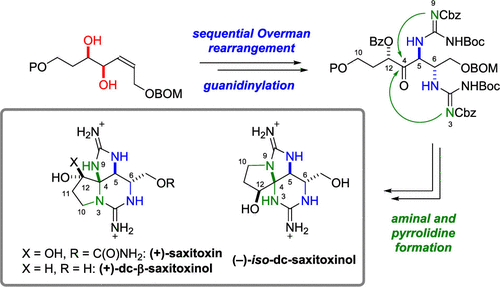
59. Unified Total Synthesis of Pentacyclic Stemoamide-type Alkaloids
Soda, Y.; Sugiyama, Y.; Yoritate, M.; Tajima, H.; Shibuya, K.; Ogihara, C.; Oishi, T.; *Sato, T., *Chida, N.
Org. Lett. 2020, 22, 7502–7507.
This article was selected as the most read articles on a monthly basis.
This article was selected as the most read articles on an annual basis.
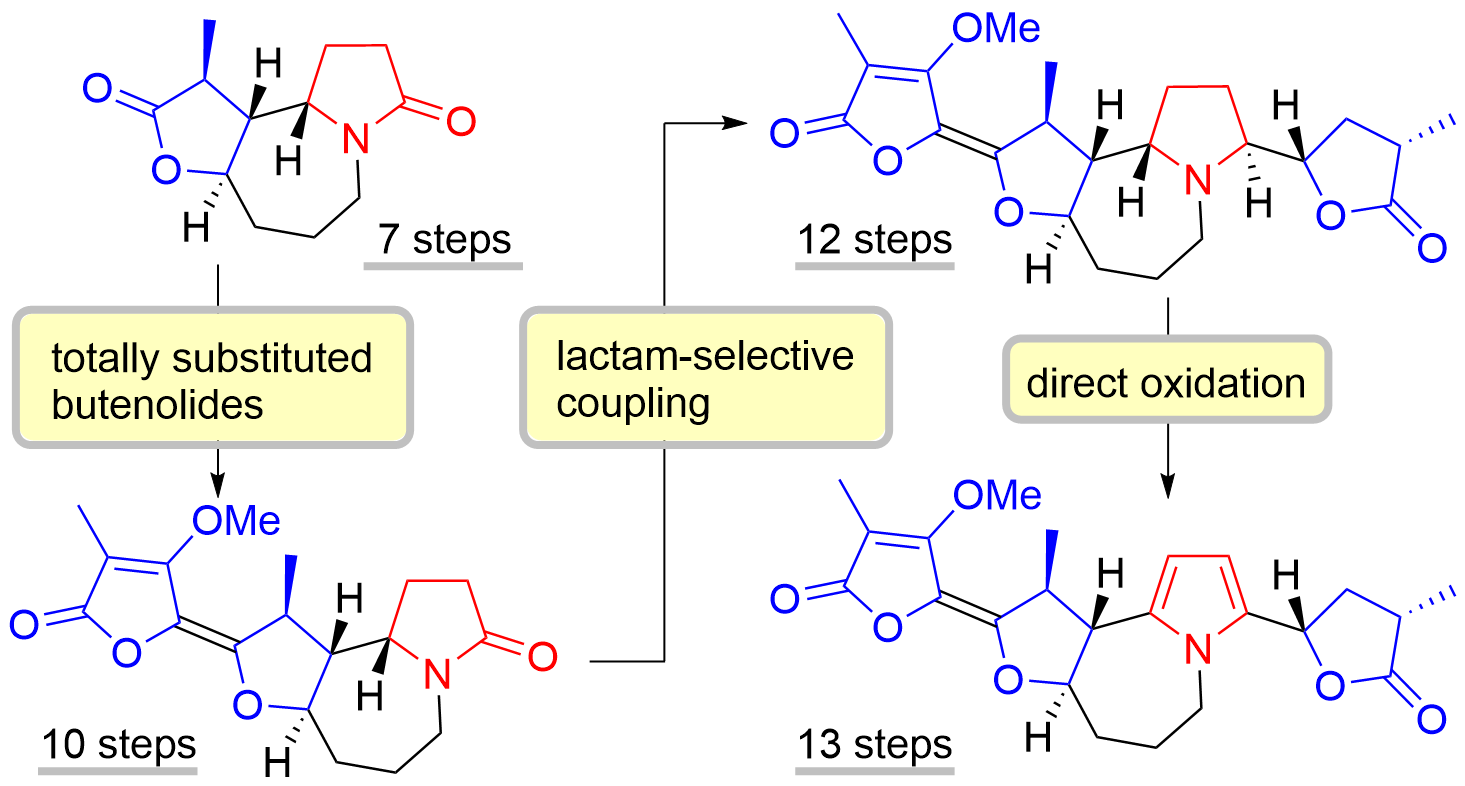
58. Copper-Catalyzed Electrophilic Etherification of Arylboronic Esters with Isoxazolidines
Katahara, S,; Takahashi, T.; Nomura, K.; *Uchiyama, M.; *Sato, T.; *Chida, N.
Chem. Asian J. 2020, 15, 1869–1872.
This article was selected as an VIP paper.
57. Enantioselective Stereodivergent Approach to α-Hydroxy Skipped Dienes: Synthesis of the Western Polyene Fragment of Corallopyronin A
Nagashima, Y.; Okada, Y.; *Sato, T.; *Chida, N.
Chem. Lett. 2019, 48, 1519–1521.
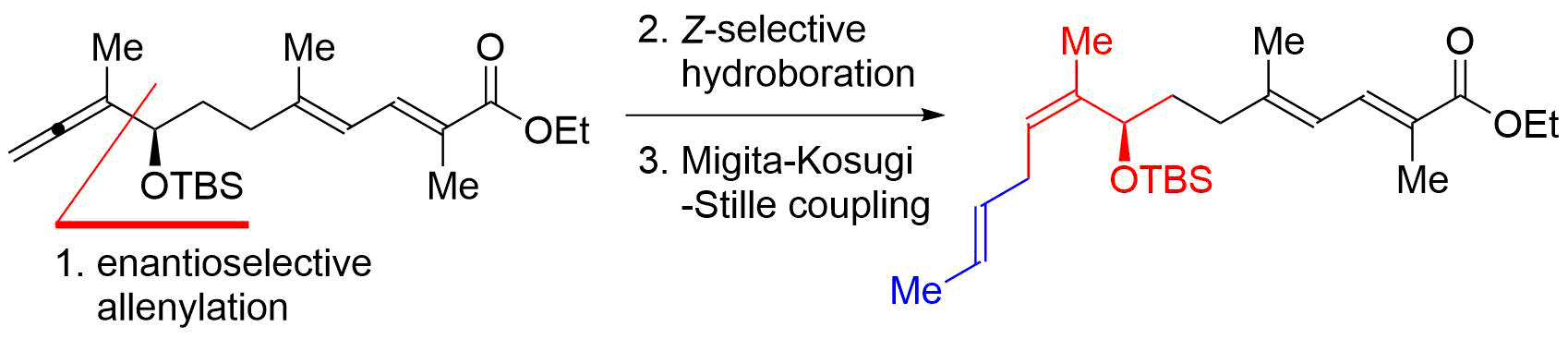
56. Iridium-Catalyzed Reductive Nucleophilic Addition to Tertiary Amides
Takahashi, Y.; Yoshii, R.; *Sato, T.; *Chida, N.
Chem. Lett. 2019, 48, 1138–1141.
This article was selected as an Editor’s Choice.
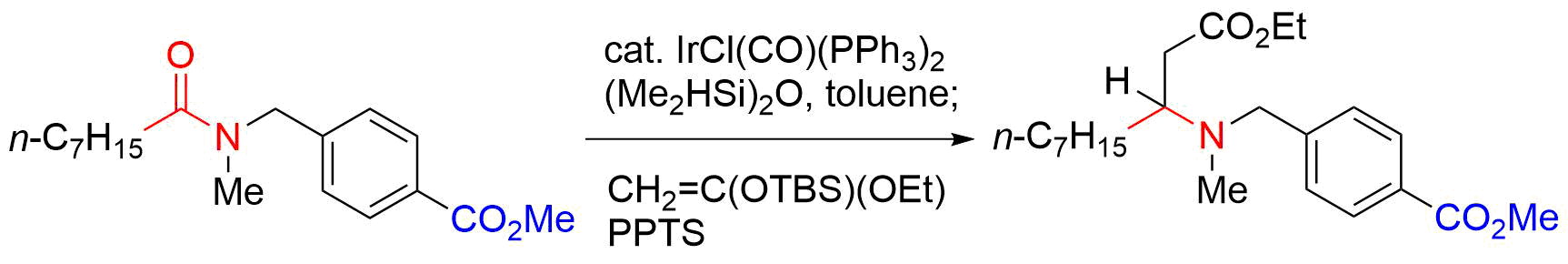
55. Copper-Catalyzed Electrophilic Amidation of Organotrifluoroborates with Use of N-Methoxyamides
Banjo, S.; B.; Nakasuji, E.; Meguro, T.; *Sato, T.; *Chida, N.
Chem. Eur. J. 2019, 25, 7941–7947.
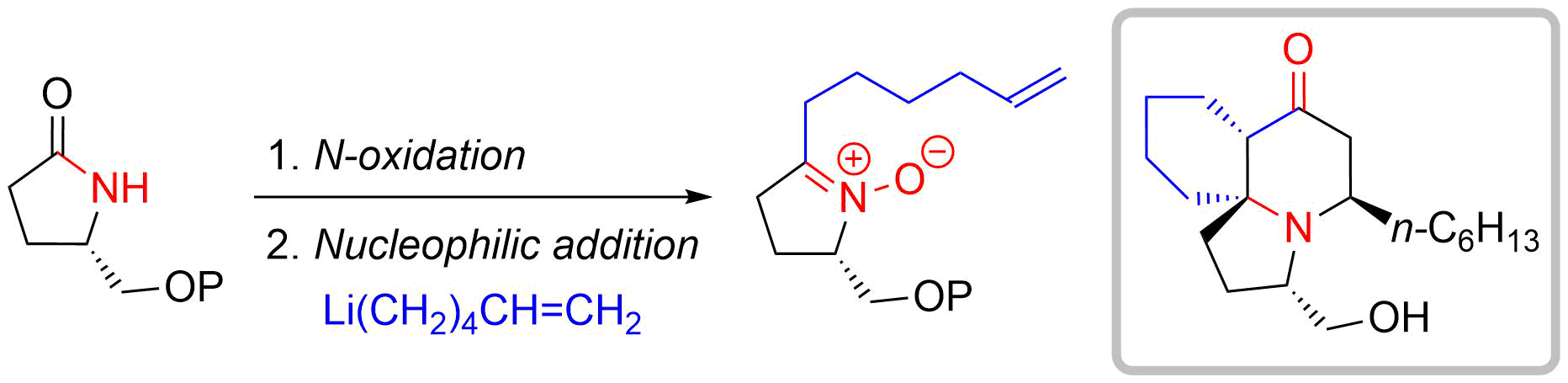
54. Nucleophilic Approach to Fully Substituted Cyclic Nitrones from N-Hydroxylactam Derivatives: Development and Application to the Total Synthesis of Cylindricine C
Hiraoka, S.; Matsumoto, T.; Matsuzaka, K.; *Sato, T.; *Chida, N.
Angew. Chem. Int. Ed. 2019, 58, 4381–4385.
This article was selected as a Hot Paper.
53. Asymmetric Total Synthesis of Fasicularin by Chiral N-Alkoxyamide Strategy
Yamamoto, S.; Komiya, Y.; Kobayashi, A.; Minamikawa, R.; Oishi, T.; *Sato, T.; *Chida, N.
Org. Lett. 2019, 21, 1868–1871.
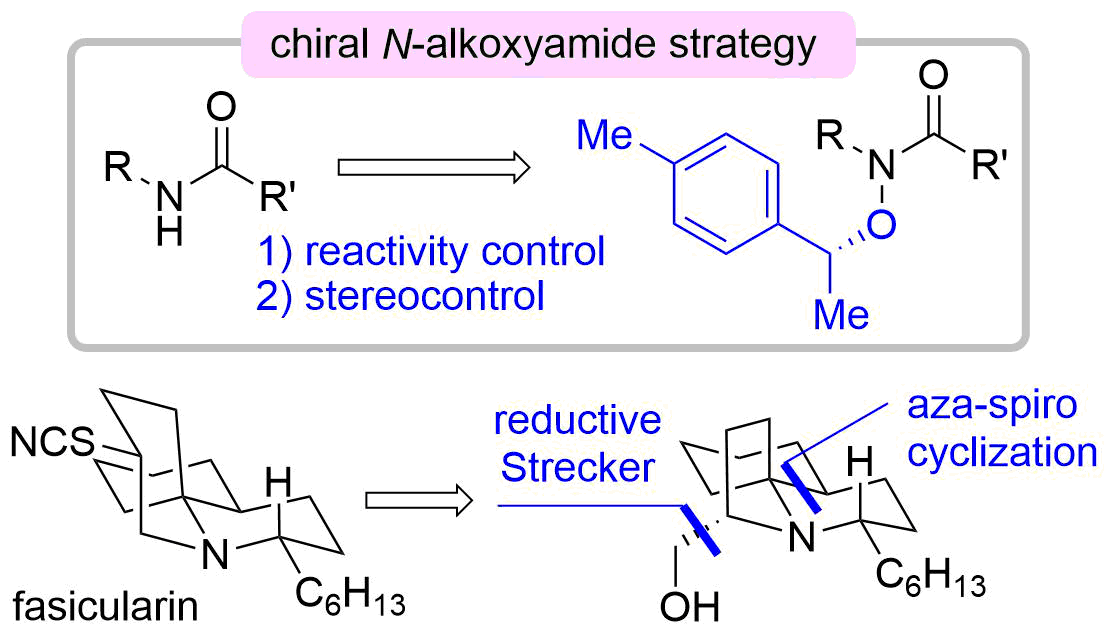
52. Unified Total Synthesis of Madangamine Alkaloids
Suto, T.; Yanagita, Y.; Nagashima, Y.; Takikawa, S.; Kurosu, Y.; Matsuo, N.; Miura, K.; Simizu, S.; *Sato, T.; *Chida, N.
Bull. Chem. Soc. Jpn. 2019, 92, 545–571.
This article was selected as Top Accessed Articles.
This article was selected as the Selected Paper.
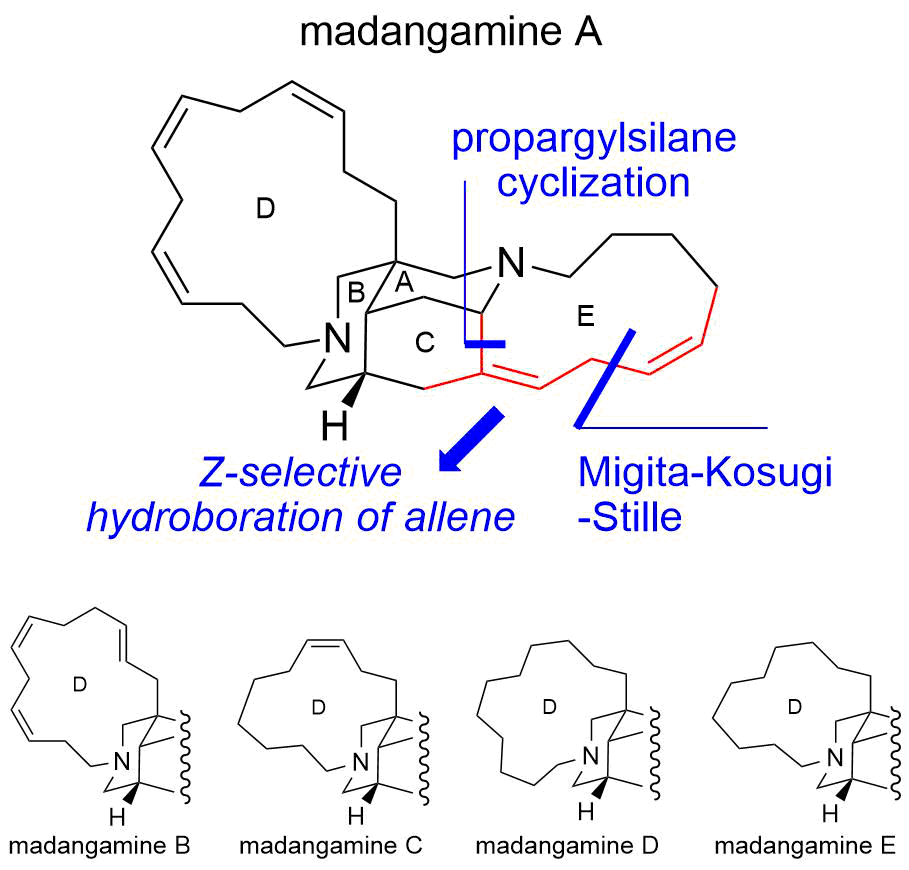
51. Total Synthesis of (–)-Zephyranthine
Ishii, K.; Seki-Yoritate, Y.; Ishibashi, M.; Liaw, M. W.; Oishi, T.; *Sato, T.; *Chida, N.
Heterocycles 2019, 99, 111–117.
This article was invited as a special issue in honor of Professor Tohru Fukuyama on 70th Birthday.
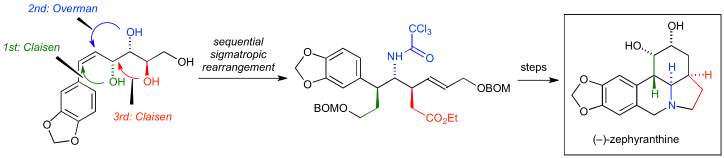
50. Iridium-Catalyzed Reductive Nucleophilic Addition to Secondary Amides
Takahashi, Y.; Yoshii, R.; *Sato, T.; *Chida, N.
Org. Lett. 2018, 20, 5705–5708.
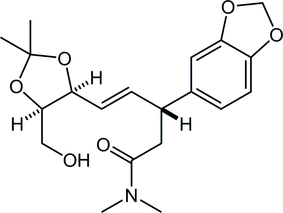
49. Crystal structure of (–)-(R,E)-3-(1,3-benzodioxol-5-yl)-5-[(4S,5R)-5-hydroxymethyl-2,2-dimethyl-1,3-dioxolan-4-yl]-N,N-dimethylpent-4-enamide
*Oishi, T.; Ishii, K.; Ishibashi, M.; Sato T.; Chida, N.
Acta Cryst. 2018, E74, 825–828.
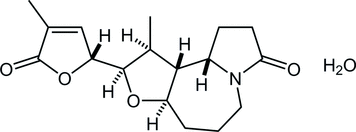
48. Crystal structure of (−)-(5R,7R,8S,9R,10S )-8-methyl-7-[(5R )- 3-methyl-2-oxooxolan-3-en-5-yl]-1-aza-6-oxatricyclo-[8.3.0.05,9]tridecan-13-one monohydrate
*Oishi, T.; Yoritate, M.; Sato T.; Chida, N.
Acta Cryst. 2018, E74, 555–558.
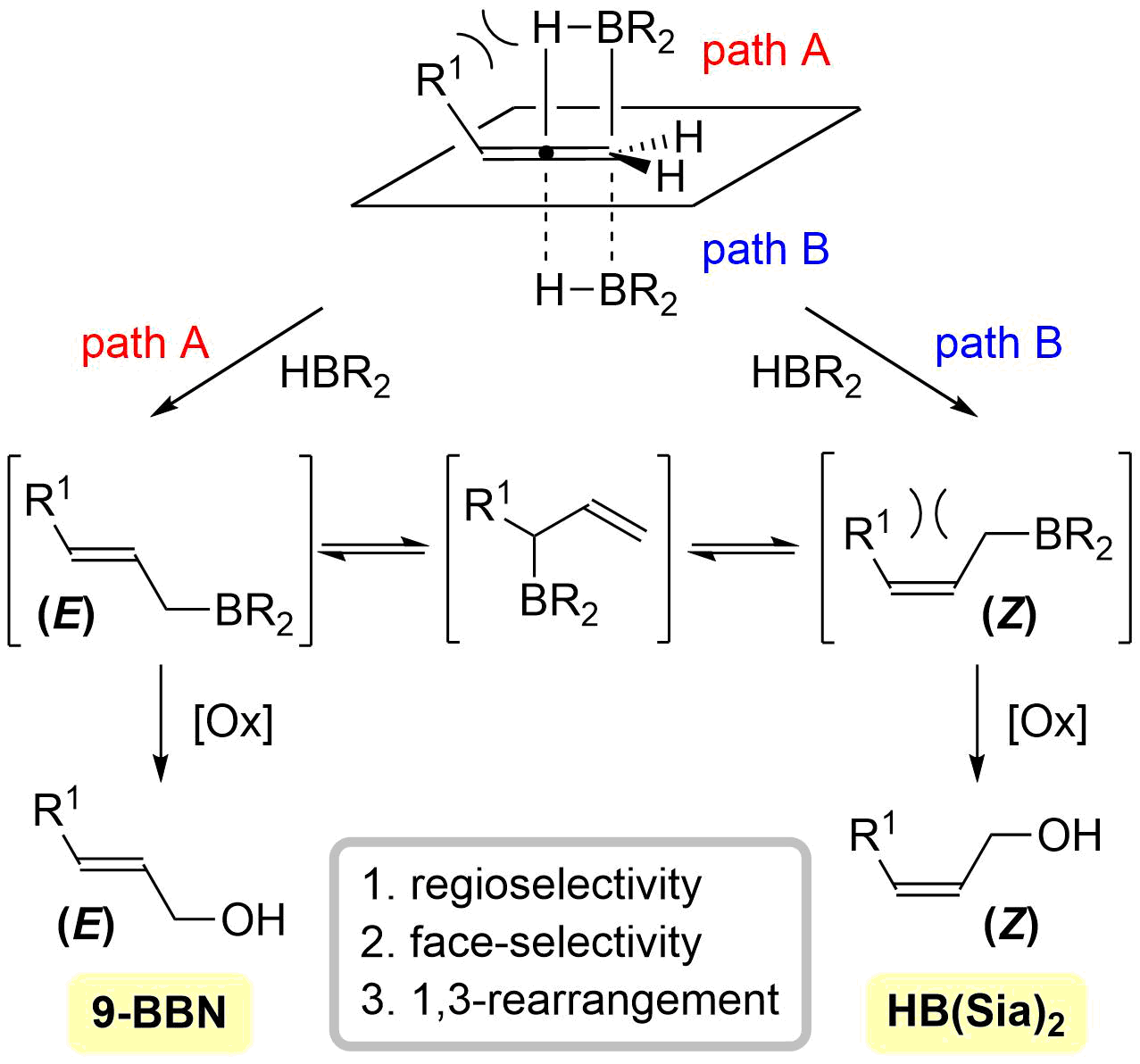
47. Stereodivergent Hydroboration of Allenes
Nagashima, Y.; Sasaki, K.; Suto, T.; *Sato, T.; *Chida, N.
Chem. Asian J. 2018, 13, 1024–1028.
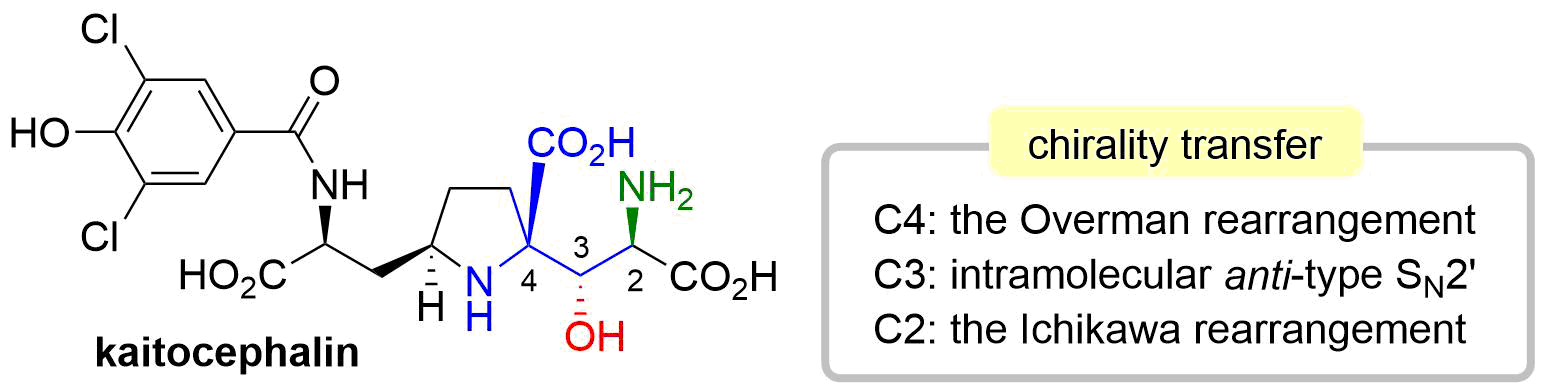
46. Synthesis of Kaitocephalin Facilitated by Three Stereoselective Allylic Transposition Reactions
Sugai, T.; Okuyama, Y.; Shin, J.; Usui, S.; Hisada, S.; Osanai, R.; Oishi, T.; *Sato, T.; *Chida, N.
Chem. Lett. 2018, 47, 454–457.
45. Synthesis of β-Hydroxy-α,α-disubstituted Amino Acids through the Orthoamide-Type Overman Rearrangement of an α,β--Unsaturated Ester and Stereodivergent Intramolecular SN2’ Reaction: Development and Application to the Total Synthesis of Sphingofungin F
Sugai, T.; Usui, S.; Tsuzaki, S.; Oishi, H.; Yasushima, D.; Hisada, S.; Fukuyasu, T.; Oishi, T.; *Sato, T.; *Chida, N.
Bull. Chem. Soc. Jpn. 2018, 91, 594–607.
This article was selected as the Selected Paper.
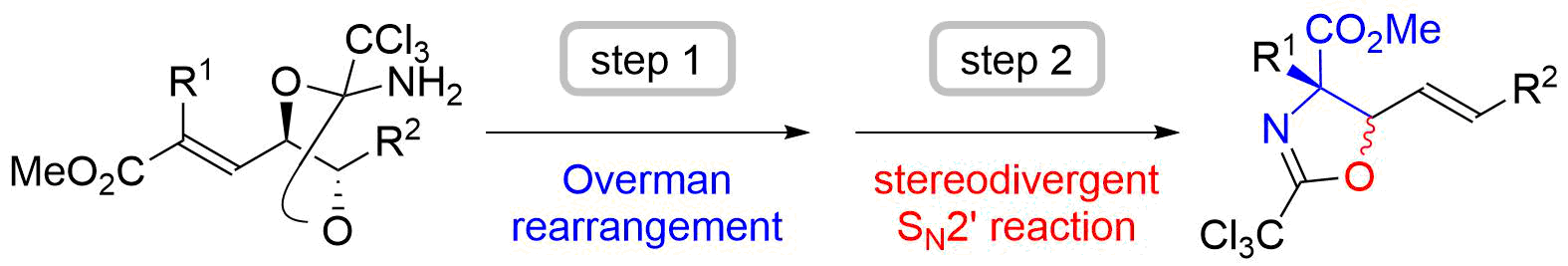
44. Unified Total Synthesis of Stemoamide-Type Alkaloids by Chemoselective Assembly of Five-Membered Building Blocks
Yoritate, M.; Takahashi, Y.; Tajima, H.; Ogihara, C.; Yokoyama, T.; Soda, Y.; Oishi, T.; *Sato, T.; *Chida, N.
J. Am. Chem. Soc. 2017, 139, 18386‒18391.
This article was selected as the most read articles on a monthly basis.
This article was highlighted in SYNFACTS (2018, 14, 231).
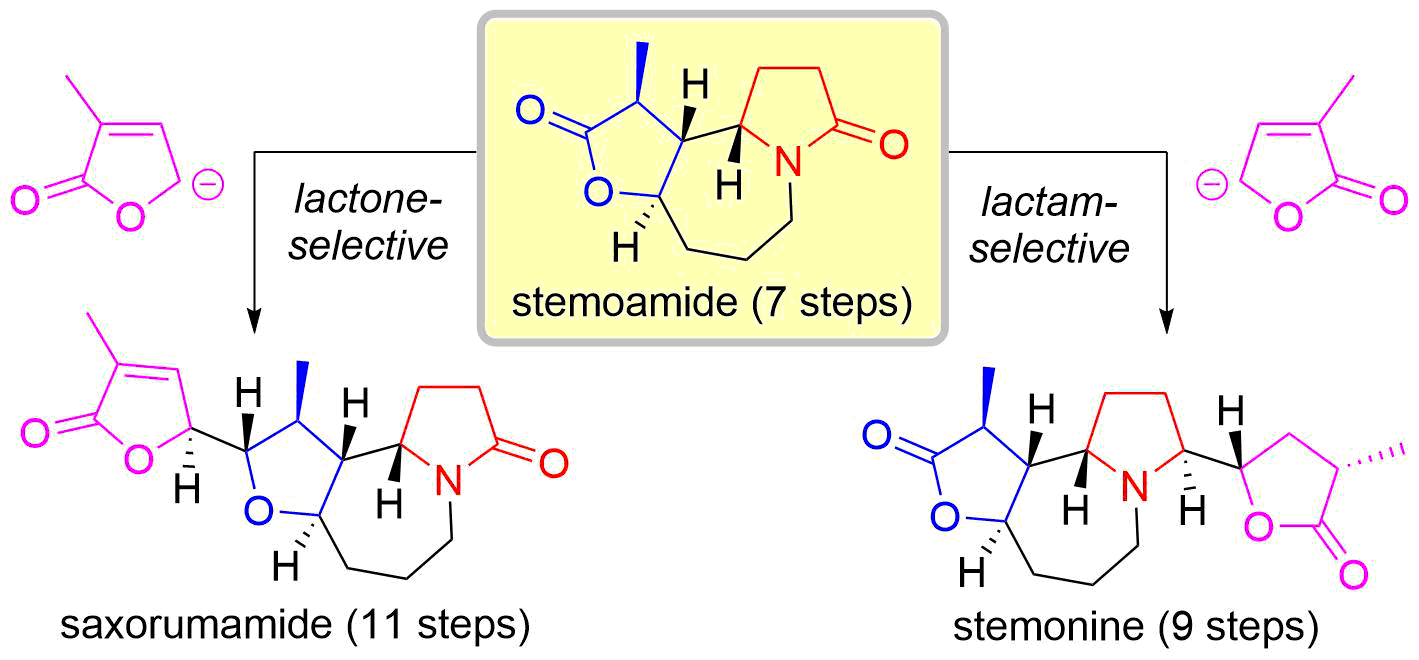
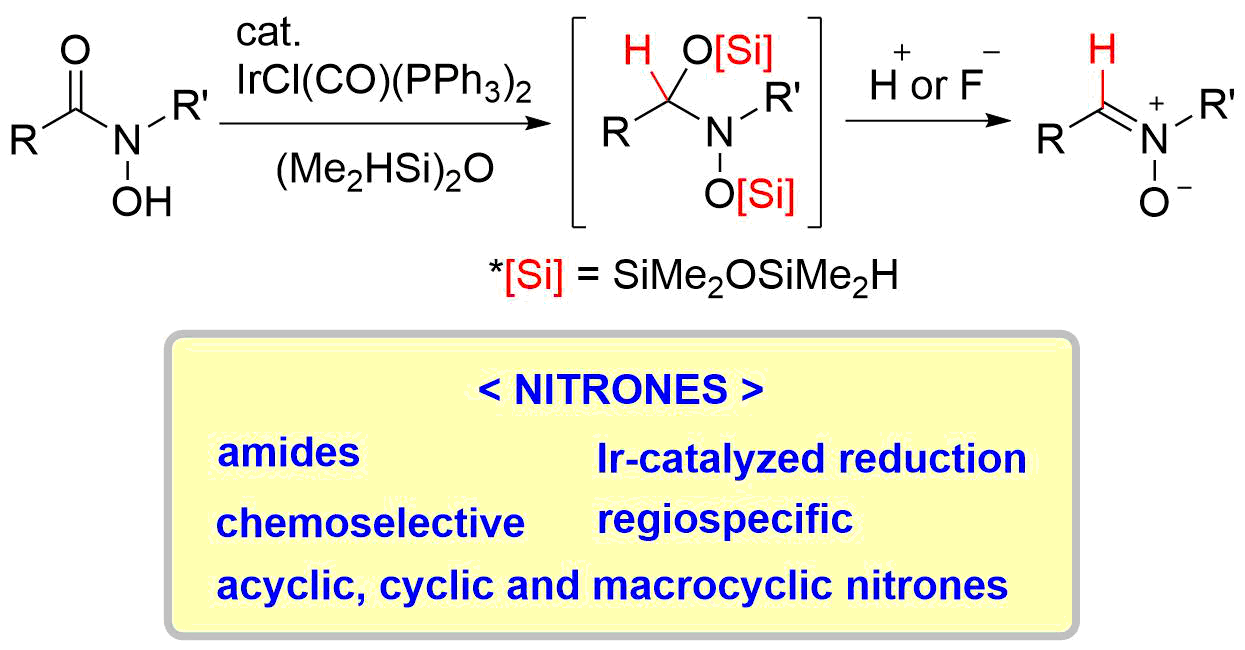
43. Reductive Approach to Nitrones from N-Siloxyamides and N-Hydroxyamides
Katahara, S.; Kobayashi, S.; Fujita, K.; Matsumoto, T.; *Sato T.; *Chida, N.
Bull. Chem. Soc. Jpn. 2017, 90, 893‒904.
This article was selected as BCSJ award.
This article was selected as a front cover.

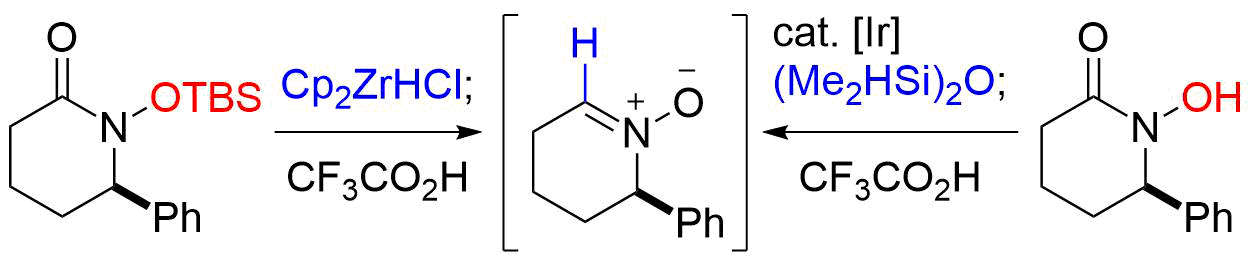
42. Crystal structure of (–)-methyl (R,E)-4-[(2R,4R )-2-amino-2-trichloromethyl-1,3-dioxolan-4-yl]-4-hydroxy-2-methylbut-2-enoate
*Oishi, T.; Kidena. M.; Sugai, T. Sato T.; Chida, N.
Acta Cryst. 2017, E73, 983–986.
41. Unified Total Synthesis of Madangamines A, C, and E
Suto, T.; Yanagita, Y.; Nagashima, Y.; Takikawa, S.; Kurosu, Y.; Matsuo, N.; *Sato T.; *Chida, N.
J. Am. Chem. Soc. 2017, 139, 2952‒2955.
This article was selected as the most read articles on a monthly basis.
This article was highlighted in SYNFACTS (2017, 13, 450).
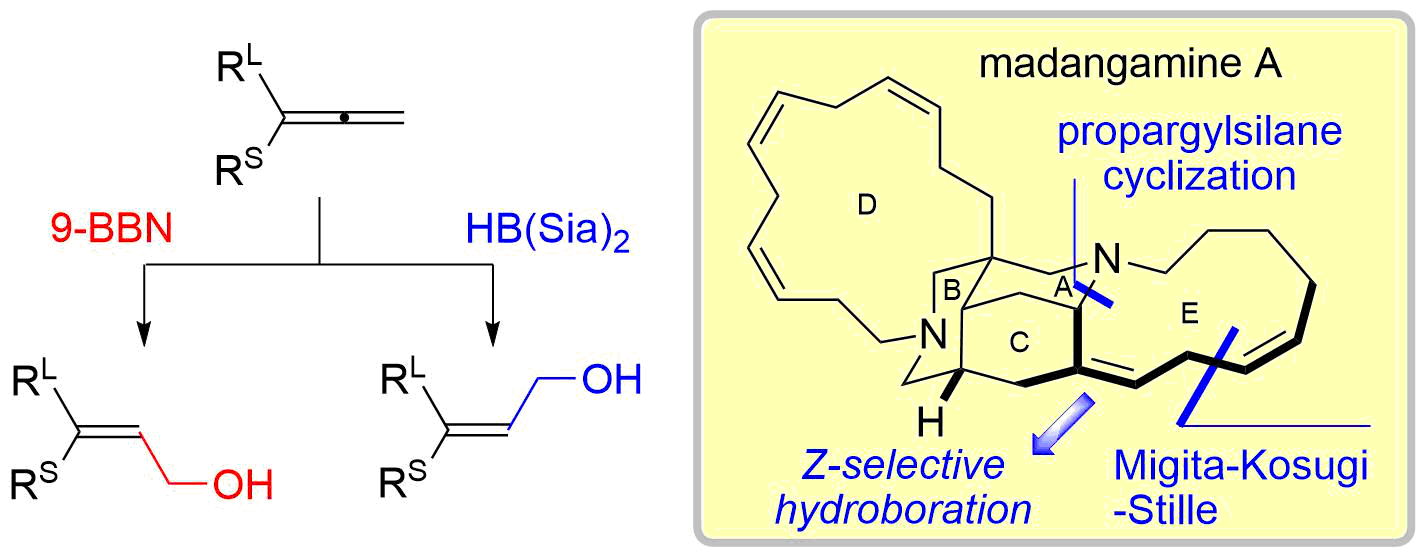
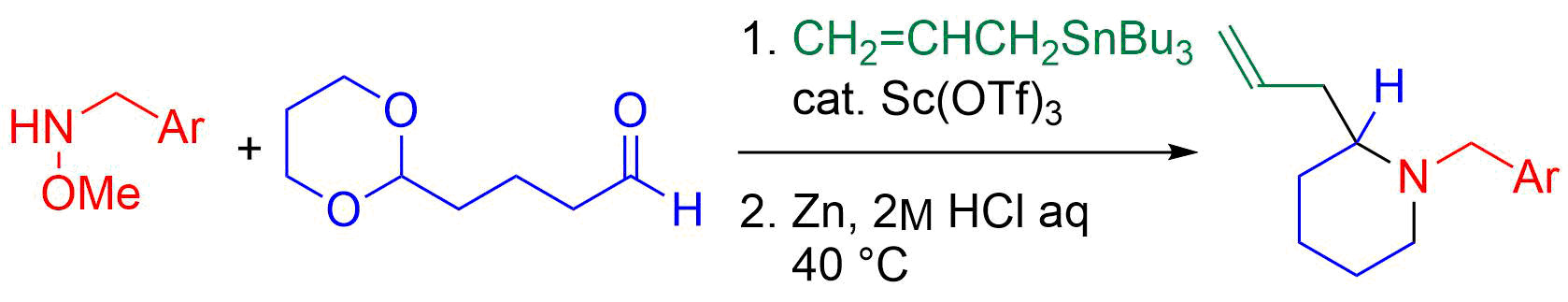
40. Copper-Catalyzed Electrophilic Amination Using N-Methoxyamines
Fukami, Y.; Wada, T.; Meguro, T.; Chida, N.; *Sato, T.
Org. Biomol. Chem. 2016, 14, 5486‒5489.
This article was invited in New Talent Themed Issue.
This article was selected as a HOT article.
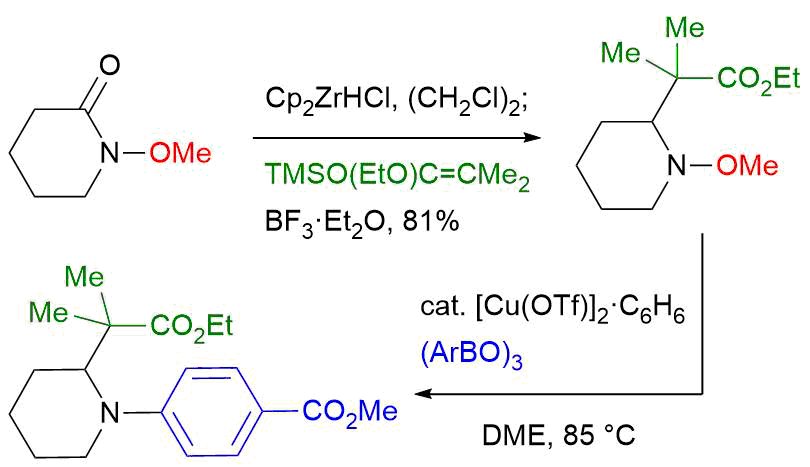
39. Crystal structure of (+)-N-[(1R,5S,6S,9S)-5-hydroxymethyl- 3,3,9-trimethyl-8-oxo-2,4,7-trioxabicyclo[4.3.0]nonan-9-yl]acetamide
*Oishi, T.; Tsuzaki, S.; Sugai, T. Sato T.; Chida, N.
Acta Cryst. 2016, E72, 756–759.
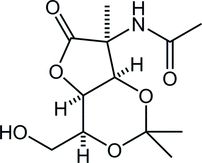
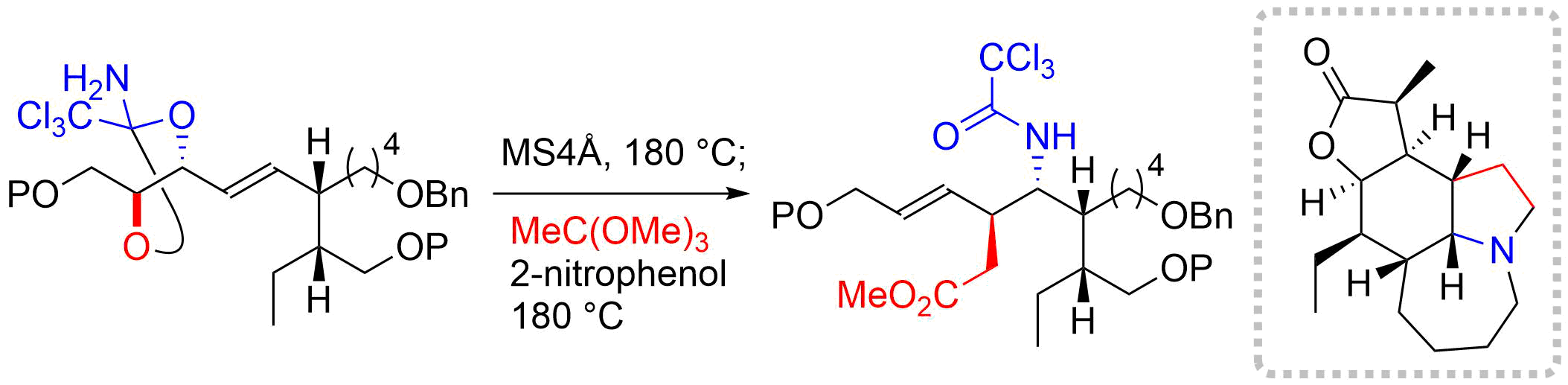
38. Total Synthesis of (–)-Stemoamide by Sequential Overman/Claisen Rearrangement
Nakayama, Y.; Maeda, Y.; Hama, N.; *Sato T.; *Chida, N.
Synthesis, 2016, 48, 1647‒1654.
This article was invited in Target Oriented Synthesis of Complex Molecules Issue.
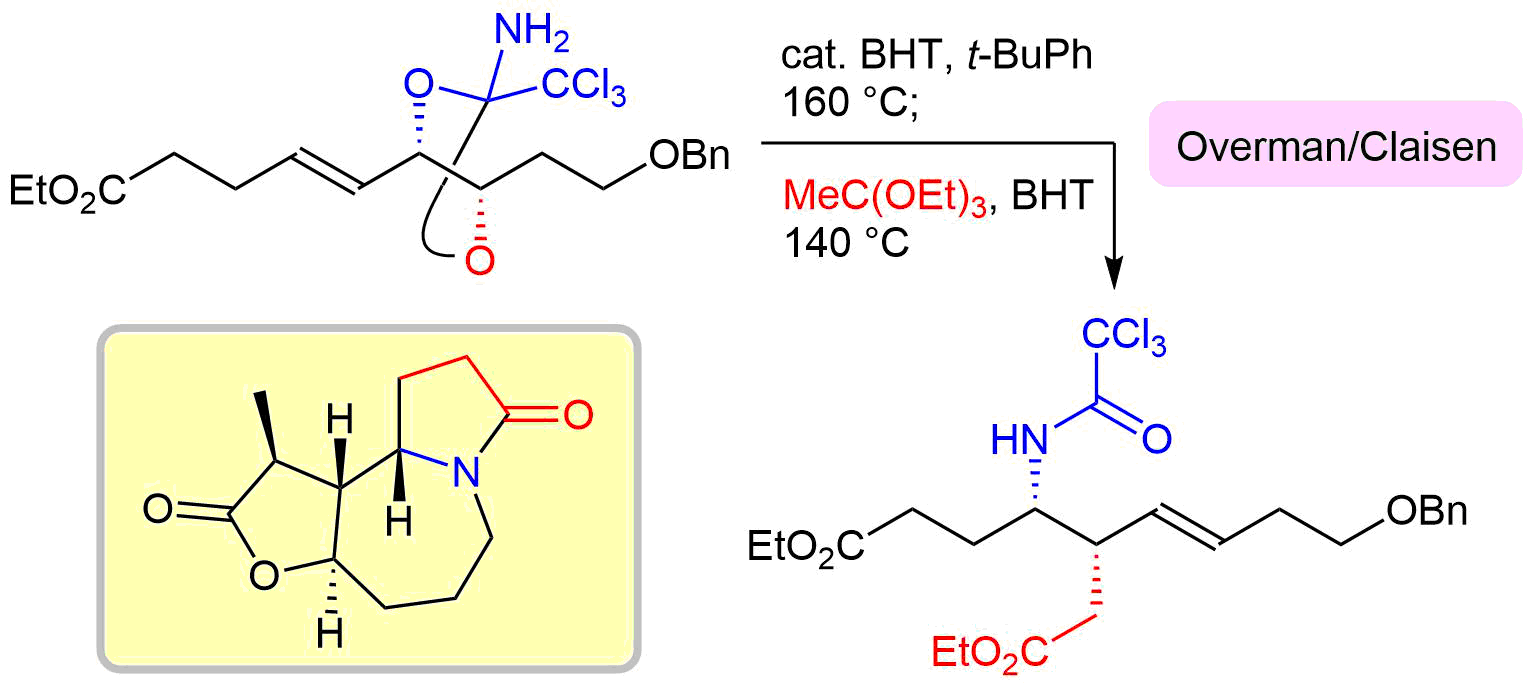
37. Crystal structure of (+)-methyl (E)-3-[(2S,4S,5R)-2-amino-5-hydroxymethyl-2-trichloromethyl-1,3-dioxolan-4-yl]-2-methylprop-2-enoate
*Oishi, T.; Yasushima, D.; Yuasa, K.; Sato T.; Chida, N.
Acta Cryst. 2016, E72, 343–346.
36. An Iridium-Catalyzed Reductive Approach to Nitrones from N-Hydroxyamides
Katahara, S.; Kobayashi, S.; Fujita, K.; Matsumoto, T.; *Sato T.; *Chida, N.
J. Am. Chem. Soc. 2016, 138, 5246‒5249.
35. Practical Synthesis of the C-ring Precursor of Paclitaxel from 3-Methoxytoluene
Fukaya, K.; Yamaguchi, Y.; Watanabe, A.; Yamamoto, H.; Sugai, S.; Sugai, T.; Sato T.; *Chida, N.
J. Antibiot. 2016, 69, 273‒279.
34. Enantioselective Total Synthesis of (+)-Neostenine
Nakayama, Y.; Maeda, Y.; Kotatsu, M.; Sekiya, R.; Ichiki, M.; *Sato, T.; *Chida, N.
Chem. Eur. J. 2016, 22, 3300‒3303.
33. Synthesis of (±)-Lasubine II Using N-Methoxyamines
Yokoyama, T.; Fukami, Y.; *Sato, T.; *Chida, N.
Chem. Asian J. 2016, 11, 470‒473.
32. Crystal structure of (±)-(5SR,6SR )-6-ethenyl-1-[(RS)-1-phenylethoxy]-1-azaspiro[4.5]decan-2-one
*Oishi, T.; Yamamoto, S.; Yokoyama, T.; Kobayashi, A.; Sato T.; Chida, N.
Acta. Cryst. 2015, E71, 1528‒1530.
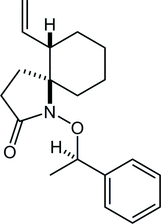
31. Crystal structure of (±)-(7RS,8SR)-7-methyl-1,4-dioxaspiro[4.5]decane-7,8-diol
*Oishi, T.; Yamamoto, H.; Sugai, T.; Fukaya, K.; Yamaguchi, Y.; Watanabe, A.; Sato T.; Chida, N.
Acta. Cryst. 2015, E71, 1181‒1184.
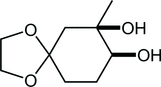
30. Synthesis of Paclitaxel. 2. Construction of the ABCD-Ring and Formal Synthesis
Fukaya, K.; Kodama, K.; Tanaka, Y.; Yamazaki, H.; Sugai, T.; Yamaguchi, Y.; Watanabe, A.; Oishi, T.; *Sato, T.; *Chida, N.
Org. Lett. 2015, 17, 2574‒2577.
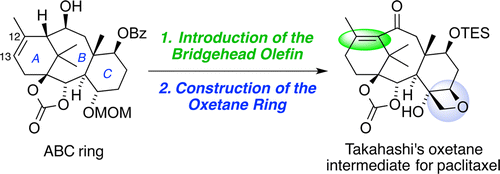
29. Synthesis of Paclitaxel. 1. Synthesis of the ABC-Ring of Paclitaxel by SmI2-Mediated Cyclization
Fukaya, K.; Tanaka, Y.; Sato, A.; Kodama, K.; Yamazaki, H.; Ishimoto, T.; Nozaki, Y.; Iwaki, Y.; Yuki, Y.; Umei, K.; Sugai, T.; Yamaguchi, Y.; Watanabe, A.; Oishi, T.; Sato, T.; *Chida, N.
Org. Lett. 2015, 17, 2570‒2573.
This article was selected as the most read articles on a monthly basis.
28. Crystal structure of (±)-(1SR,5SR,6SR,7SR,10SR,11SR,13SR)-13-benzyloxy-7-methoxymethoxy-11,15,18,18-tetramethyl-3-oxo-2,4-dioxatetracyclo[12.3.1.01,5.06,11]octadeca-14,16-dien-10-ylbenzoate
*Oishi, T.; Fukaya, K.; Yamaguchi, Y.; Sugai, T., Watanabe, A.; Sato T.; Chida N.
Acta. Cryst. 2015, E71, 490‒493.
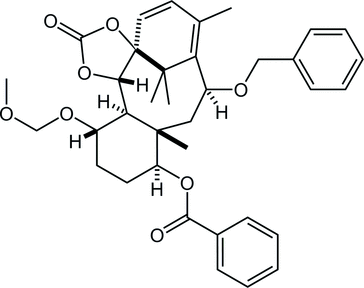
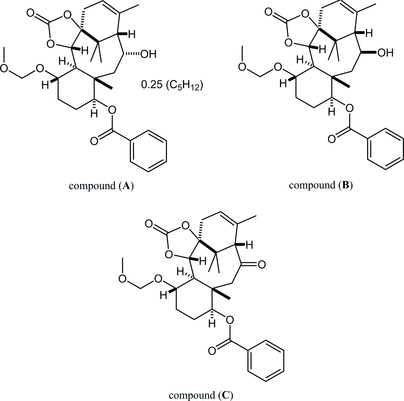
27. Crystal structures of (±)-(1SR,5SR,6SR,7SR,10SR,11SR,13RS,14SR)-13-hydroxy-7-methoxymethoxy-11,15,18,18-tetramethyl-3-oxo-2,4-dioxatetracyclo[12.3.1.01,5.06,11]octadec-15-en-10-yl benzoate, its 13-epimer and 13-one derivative
*Oishi, T.; Fukaya, K.; Yamaguchi, Y.; Sugai, T., Watanabe, A.; Sato T.; Chida N.
Acta. Cryst. 2015, E71, 466‒472.
26. Synthesis of Diazatricyclic Common Structure of Madangamine Alkaloids
Yanagita, Y.; Suto, T.; Matsuo, N.; Kurosu, Y.; *Sato T.; *Chida, N.
Org. Lett. 2015, 17, 1946‒1949.
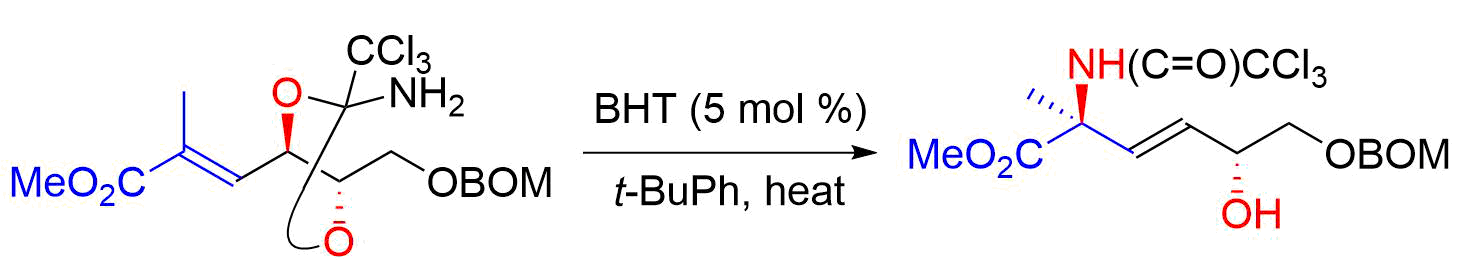
25. Total Synthesis of Sphingofungin F by Orthoamide-Type Overman Rearrangement of an Unsaturated Ester
Tsuzaki, S.; Usui, S.; Oishi, H.; Yasushima, D.; Fukuyasu, T.; Oishi, T.; *Sato T.; *Chida, N.
Org. Lett. 2015, 17, 1704‒1707.
24. Iridium-Catalyzed Chemoselective Reductive Nucleophilic Addition to N-Methoxyamides
Nakajima, M.; *Sato T.; *Chida, N.
Org. Lett. 2015, 17, 1696‒1699.
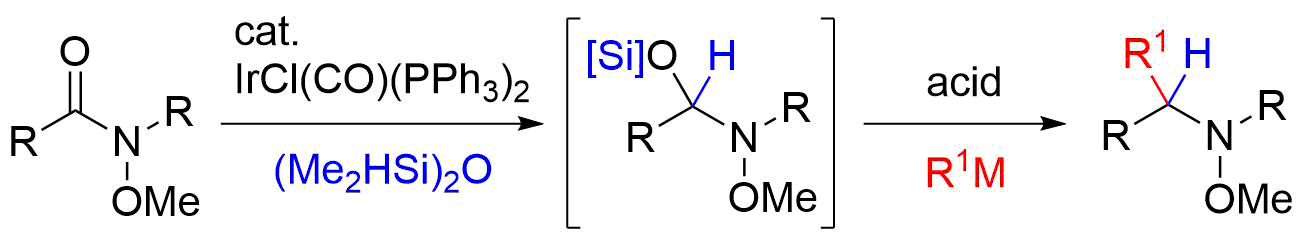
23. Total Syntheses of (±)-Gephyrotoxin and (±)-Perhydrogephyrotoxin
Shirokane, K.; Tanaka, Y.; Yoritate, M.; Takayama, N.; *Sato T.; *Chida, N.
Bull. Chem. Soc. Jpn. 2015, 88, 522‒537.
This article was selected as BCSJ award.
This article was selected as a front cover.

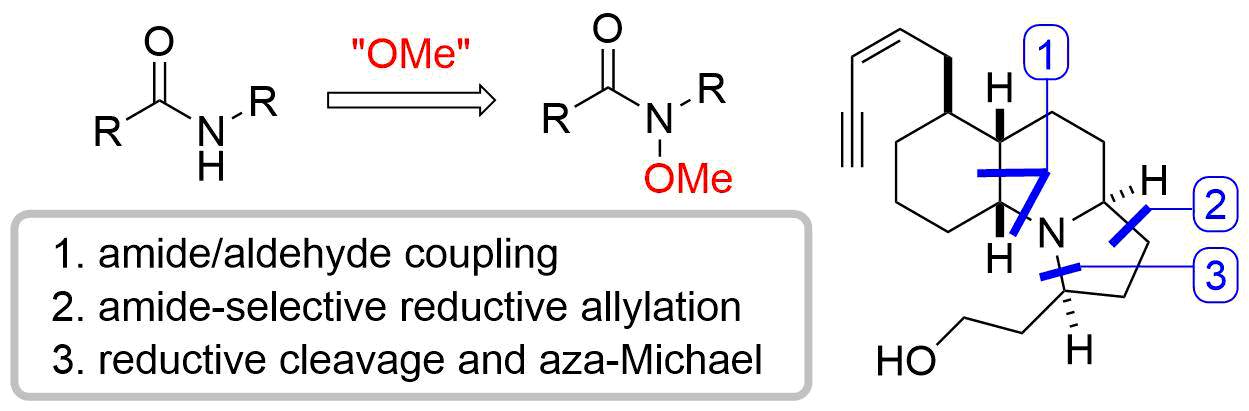
22. Crystal structure of (±)-(4RS,5RS,7SR)-4-[(1RS,2RS,3RS,6RS)-3-benzoyloxy-2-(2-hydroxyethyl)-6-methoxymethoxy-2-methylcyclohexyl]-8,10,10-trimethyl-2-oxo-1,3-dioxaspiro[4.5]dec-8-en-7-yl benzoate benzene monosolvate
*Oishi, T.; Yamaguchi, Y.; Fukaya, K.; Sugai, T., Watanabe, A.; Sato T.; Chida N.
Acta. Cryst. 2015, E71, 8‒11.
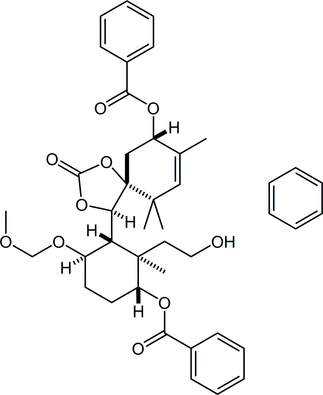
21. Chemoselective Reductive Nucleophilic Addition to Tertiary Amides, Secondary Amides and N-Methoxyamides
Nakajima, M.; Oda, Y.; Wada, T.; Minamikawa, R.; Shirokane, K.; *Sato, T.; *Chida, N.
Chem. Eur. J. 2014, 20, 17565‒17571.
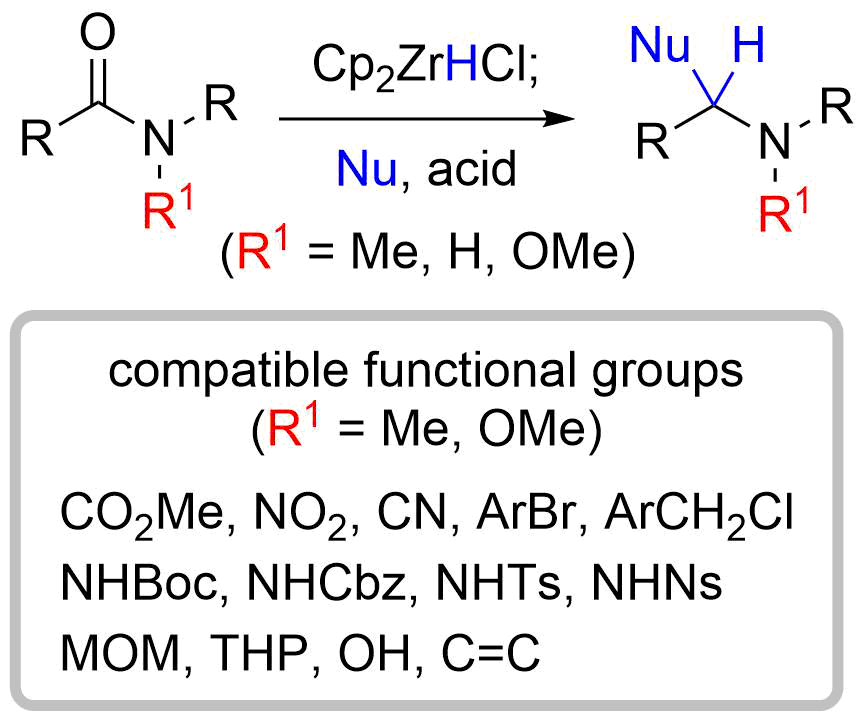
20. (5R*)-5-[(2S*,5S*)-1-Methoxy-5-phenylpyrrolidin-2-yl]- 3-methylfuran-2(5H)-one
*Oishi, T.; Yoritate, M.; Sato, T.; Chida, N.
Acta Cryst. 2014, E70, o839.
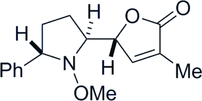
19. Two-step Synthesis of Multi-Substituted Amines by Using an N-Methoxy Group as a Reactivity Control Element
Yoritate, M.; Meguro, T.; Matsuo, N.; Shirokane, K.; *Sato, T.; *Chida, N.
Chem. Eur. J. 2014, 20, 8210‒8216.
This article was selected as VIP (Very Important Paper).
This article was selected as a front cover.

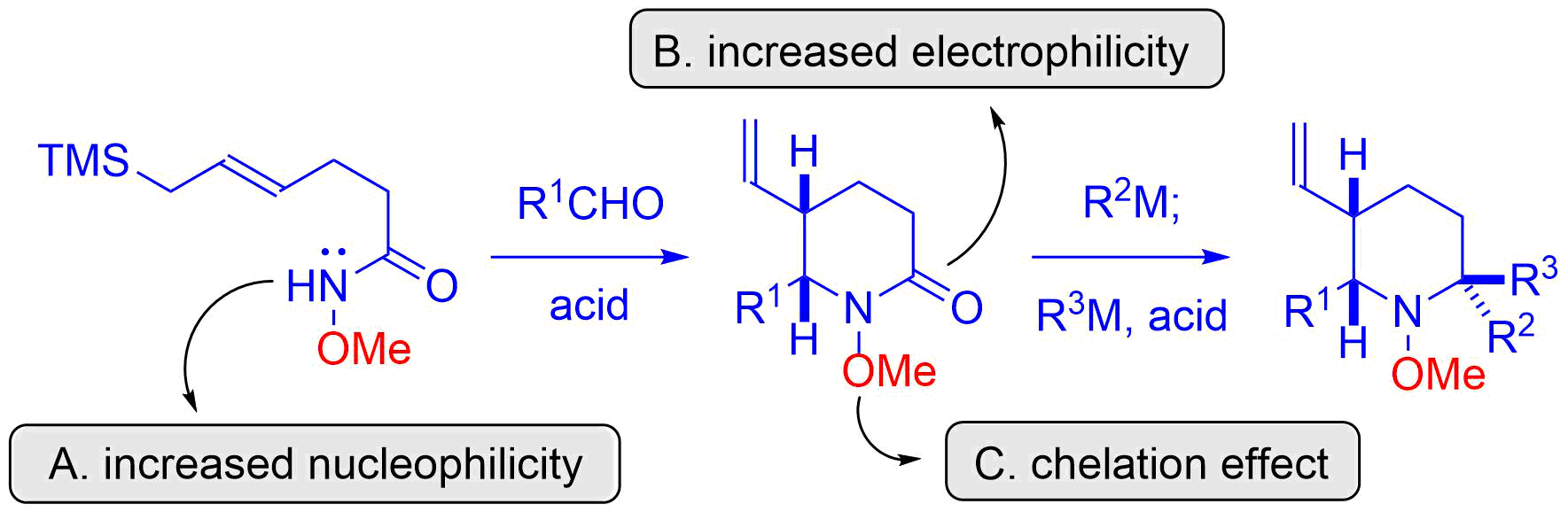
18. Total Synthesis of (±)-Gephyrotoxin by Amide-Selective Reductive Nucleophilic Addition
Shirokane, K.; Wada, T.; Yoritate, M.; Minamikawa, R.; Takayama, N.; *Sato T.; *Chida, N.
Angew. Chem. Int. Ed. 2014, 53, 512‒516.
This article was selected as VIP (Very Important Paper).
This article was selected as the most accessed articles in 12/2013.
This article was selected as the most accessed articles in 11/2013-10/2014.
This article was highlighted in SYNFACTS (2014, 10, 116).
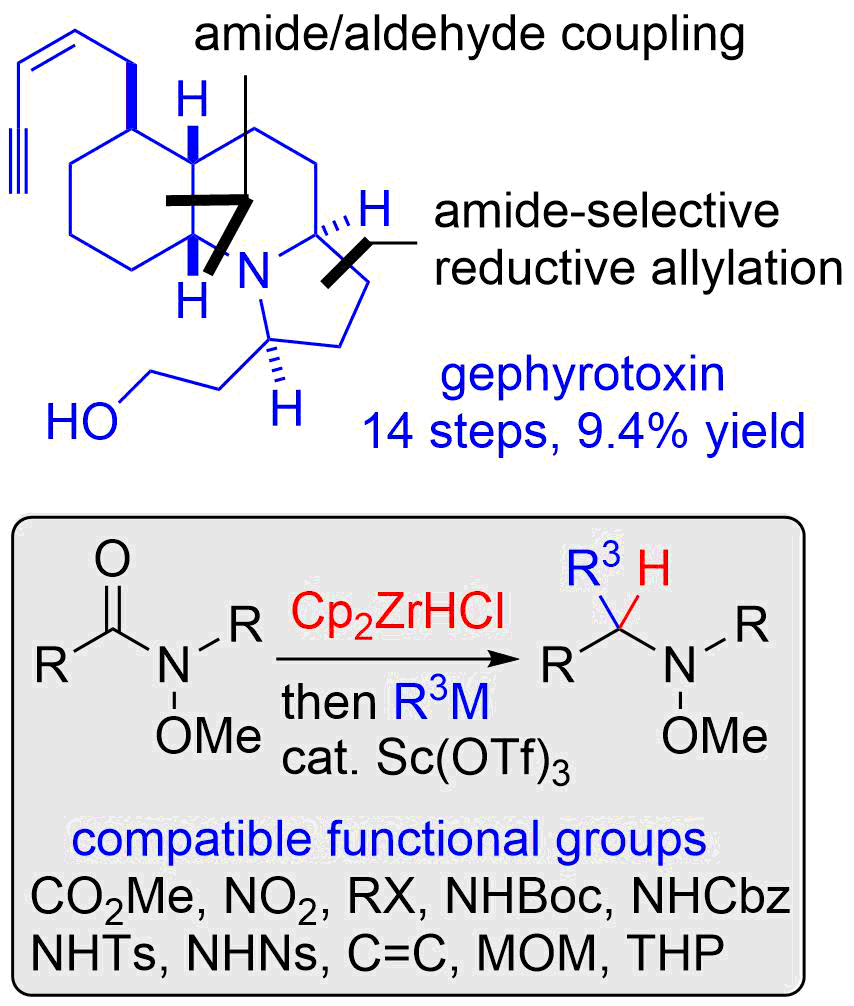
17. Cascade- and Orthoamide-type Overman Rearrangements of Allylic Vicinal Diols
Nakayama, Y.; Sekiya, R.; Oishi, H.; Hama, N.; Yamazaki, M.; *Sato, T.; *Chida, N.
Chem. Eur. J. 2013, 19, 12052‒12058.
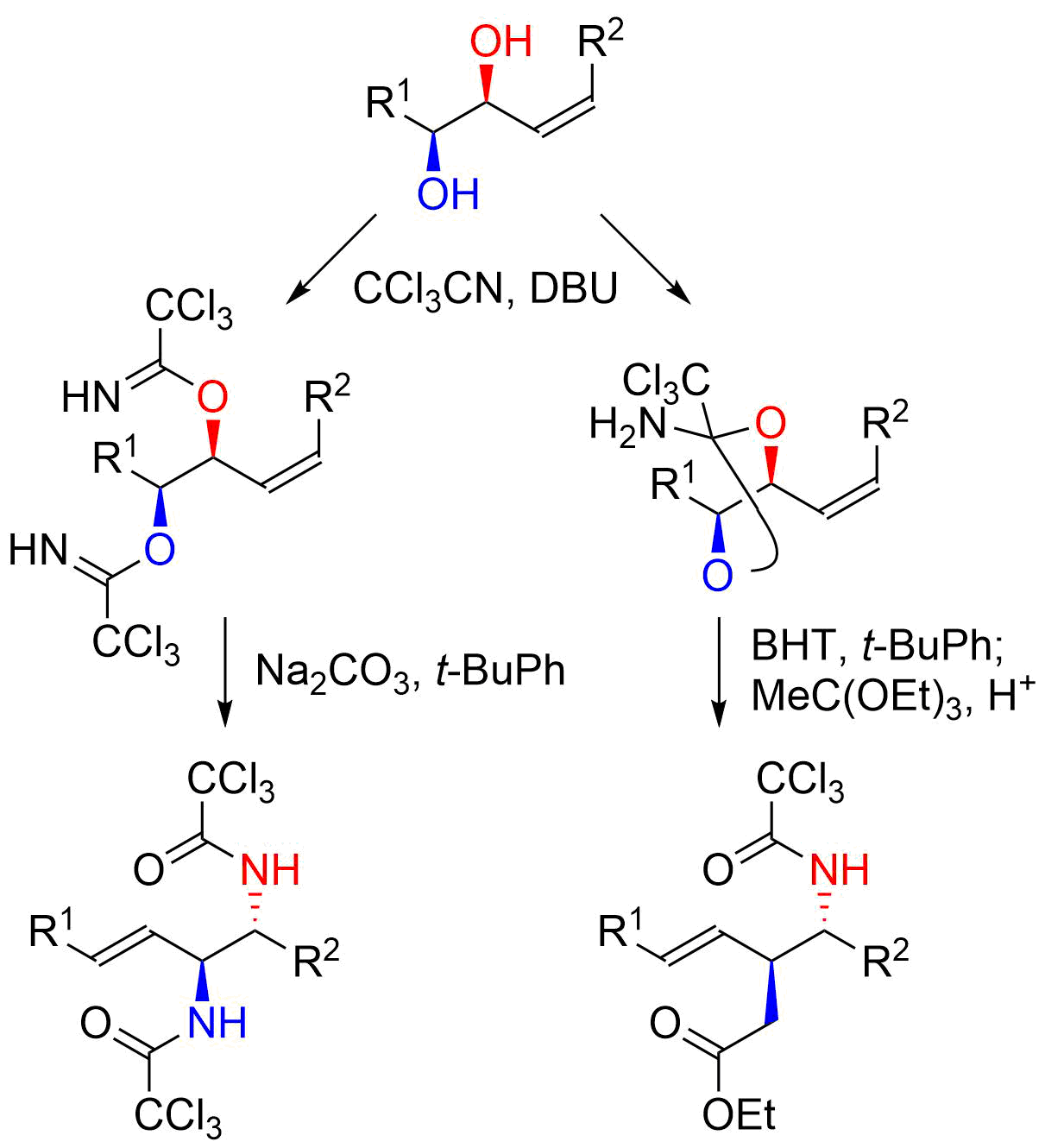
16. Direct Nucleophilic Addition to N-Alkoxyamides
Yanagita, Y.; Nakamura, H.; Shirokane, K.; Kurosaki, Y.; *Sato, T.; *Chida, N.
Chem. Eur. J. 2013, 19, 678‒684.
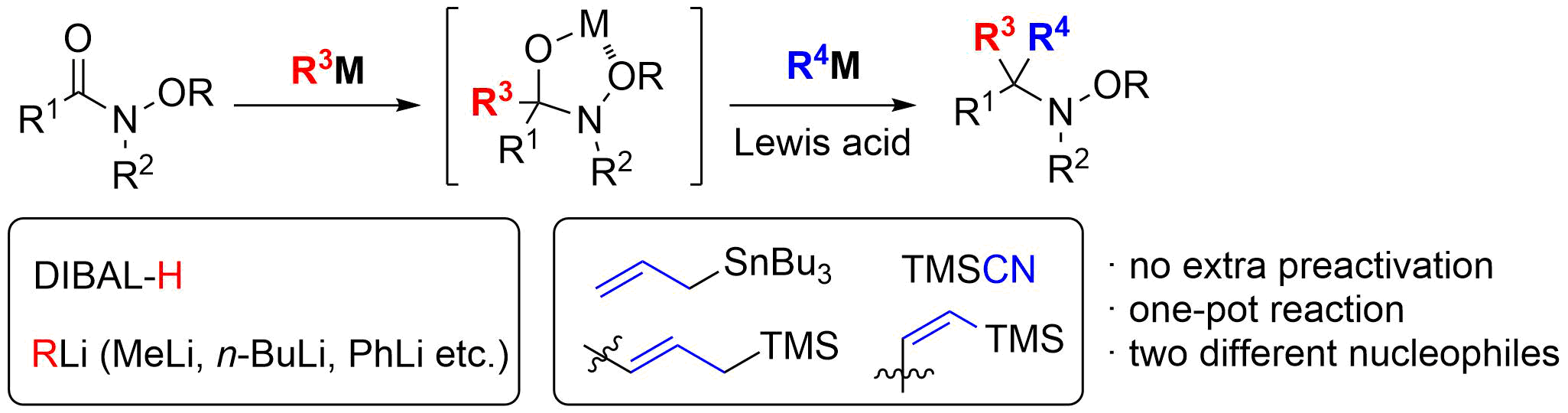
15. Synthesis of (‒)-Morphine: Application of Sequential Claisen/Claisen Rearrangement of an Allylic Vicinal Diol
Ichiki, M.; Tanimoto, H.; Miwa, S.; Saito, R.; Sato, T.; *Chida, N.
Chem. Eur. J. 2013, 19, 264‒269.
This article was selected as the most read article Top 25 on an annually basis.
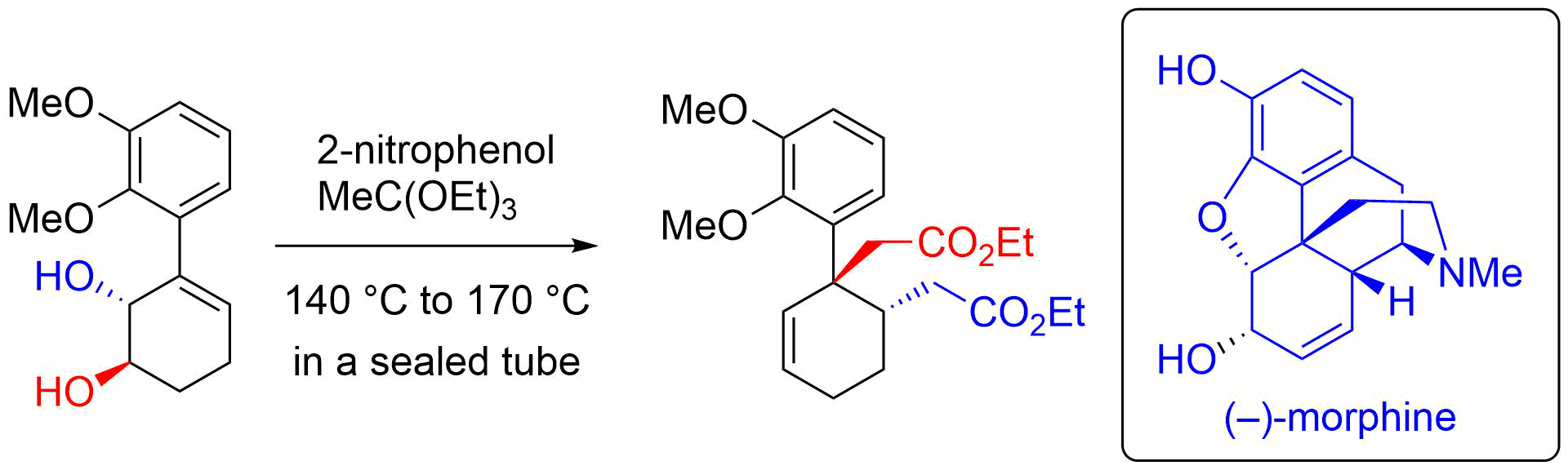
14. (+)-(1S,5R,6R)-6-[(S)-1-Hydroxy-2-(methoxymethyloxy)ethyl]-1-methyl-3-trichloromethyl-2-aza-4,7-dioxabicyclo[3.3.0]oct-2-en-8-one
*Oishi, T.; Oishi, H.; Tsuzaki, S.; Sato, T.; Chida, N.
Acta Cryst. 2012, E68, o3185.
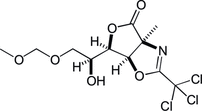
13. Chirality Transfers through Sequential Sigmatropic Rearrangements of Allylic Vicinal Diols: Development and Application to Total Synthesis of (–)-Kainic Acid
Kitamoto, K.; Nakayama, Y.; Sampei, M.; Ichiki, M.; Furuya, N.; *Sato, T.; *Chida, N.
Eur. J. Org. Chem. 2012, 4217‒4231.
12. Concise Synthesis of α-Trisubstituted Amines from Ketones Using N-Methoxyamines”
Kurosaki, Y.; Shirokane, K.; Oishi, T.; *Sato, T.; *Chida, N.
Org. Lett. 2012, 14, 2098‒2101.
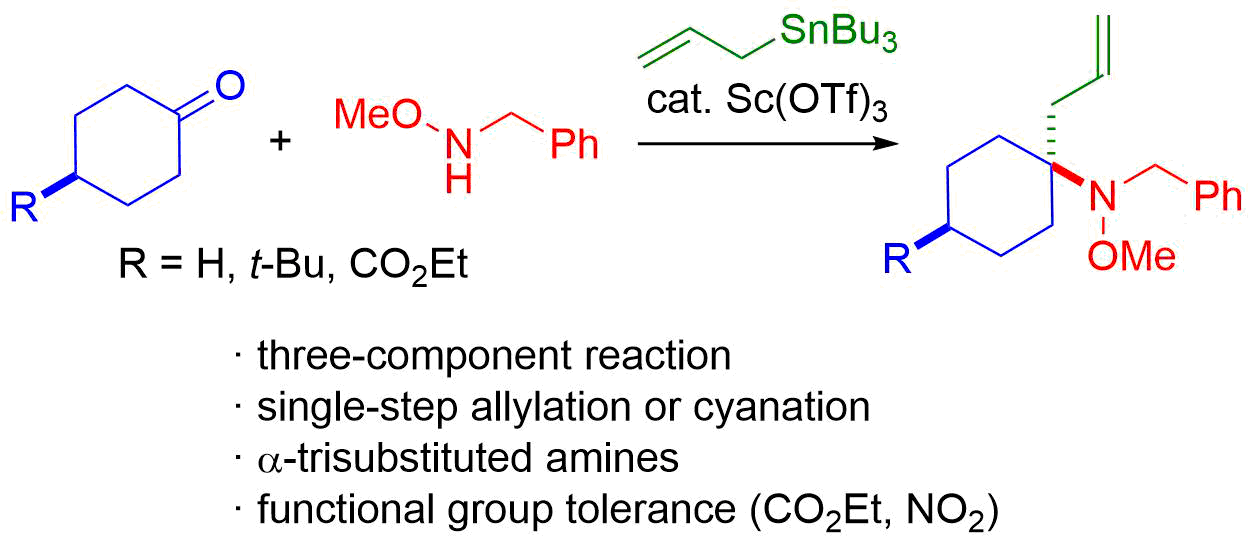
11. Direct Chemoselective Allylation of Inert Amide Carbonyls
Oda, Y.; *Sato, T.; *Chida, N.
Org. Lett. 2012, 14, 950‒953.
This article was highlighted in SYNFACTS (2012, 14, 428).
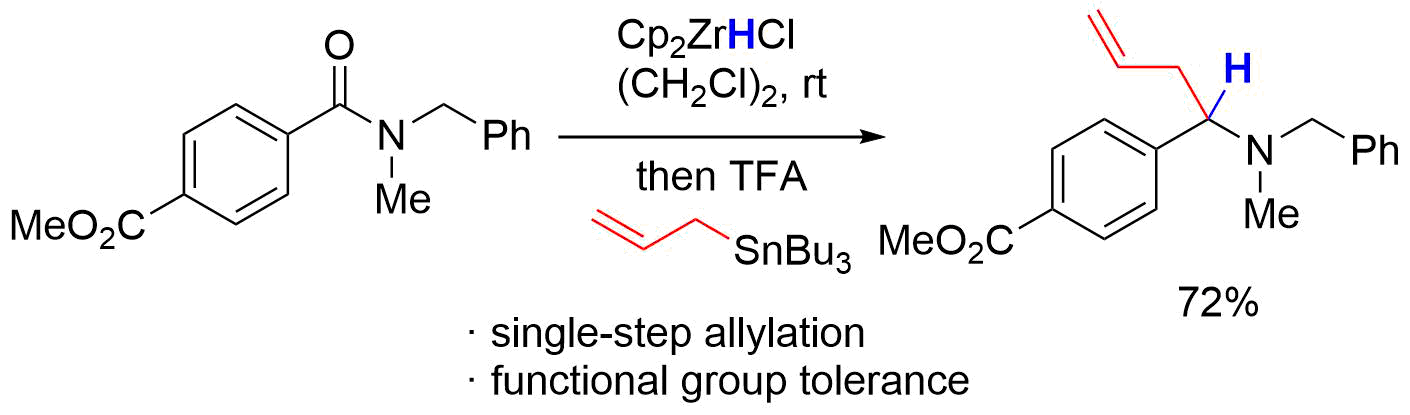
10. Total Synthesis of Broussonetine F: The Orthoamide Overman Rearrangement of an Allylic Diol
Hama, N.; Aoki, T.; Miwa, S.; Yamazaki, M.; Sato, T.; *Chida, N.
Org. Lett. 2011, 13, 616‒619.
This article was selected as the most read articles on a monthly basis.
This article was selected as the most read articles for quarter 1, 2011.
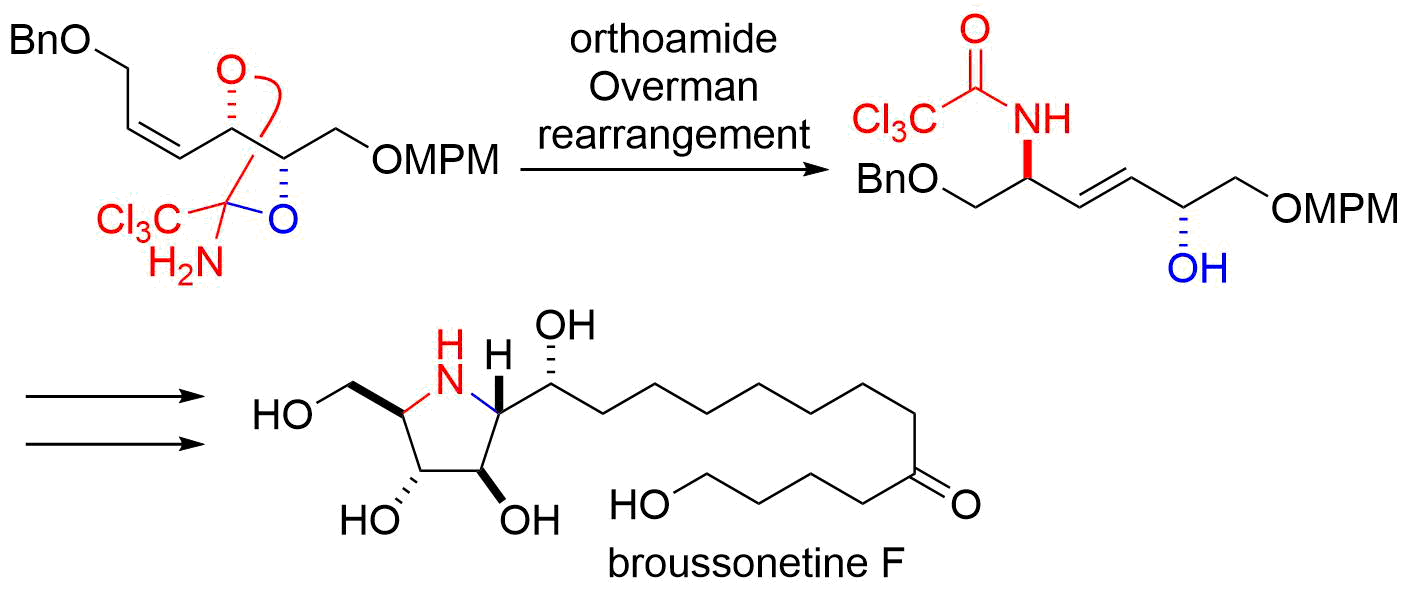
9. Total Synthesis of (‒)-Salinosporamide A
Kaiya, Y.; Hasegawa, J.; Momose, T.; *Sato, T.; *Chida, N.
Chem. Asian J. 2011, 5, 209‒219.
This article was selected as the most read article Top 25 on an annually basis.
8. Novel Sequential Sigmatropic Rearrangements of Allylic Diols: Application to the Total Synthesis of (‒)-Kainic Acid
Kitamoto, K.; Sampei, M.; Nakayama, Y.; *Sato, T.; *Chida, N.
Org. Lett. 2010, 12, 5756‒5759.
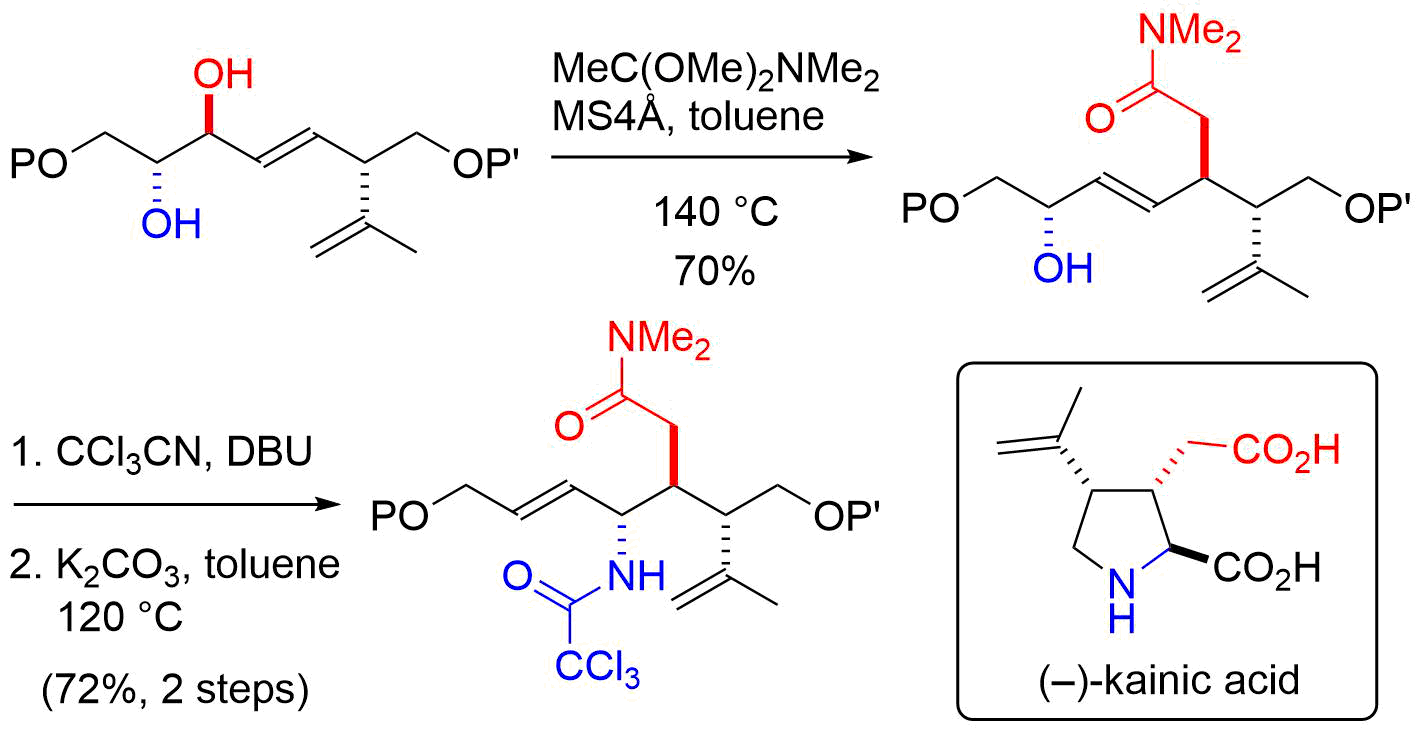
7. A Direct Entry to Substituted N-Methoxyamines from N-Methoxyamides via N-Oxyiminium Ions
Shirokane, K.; Kurosaki, Y.; *Sato, T.; *Chida, N.
Angew. Chem. Int. Ed. 2010, 49, 6369‒6372.
This article was highlighted in SYNFACTS (2010, 12, 1412).
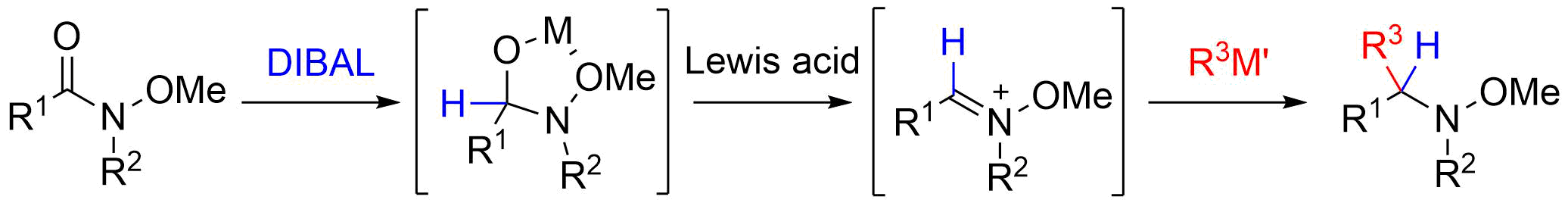
6. Formal Synthesis of Salinosporamide A Starting from D-Glucose
Momose, T.; Kaiya, Y.; Hasegawa, J.; Sato, T.; *Chida, N.
Synthesis, 2009, 2983‒2991.
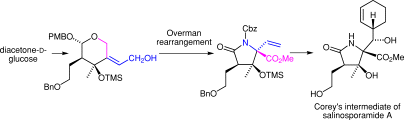
5. Total Synthesis of (‒)-Agelastatin A: The Application of a Sequential Sigmatropic Rearrangement
Hama, N.; Matsuda, T.; Sato, T.; *Chida, N.
Org. Lett. 2009, 11, 2687‒2690.
This article was highlighted in SYNFACTS (2009, 12, 1314).
4. Construction of Epidithiodioxopiperazines by Directed Oxidation of Hydroxyproline-Derived Dioxopiperazines
*Overman, L. E.; Sato, T.
Org. Lett. 2007, 9, 5267‒5270.
3. Total Synthesis and Bioactivity of an Unnatural Enantiomer of Merrilactone A: Development of an Enantioselective Desymmetrization Strategy
*Inoue, M.; Lee, N.; Kasuya, S.; Sato, T.; Hirama, M.; Moriyama, M.; Fukuyama, Y.
J. Org. Chem. 2007, 72, 3065–3075.
2. Asymmetric Total Synthesis of (‒)-Merrilactone A: Use of a Bulky Protecting Group as Long-Range Stereocontrolling Element
*Inoue, M.; Sato, T.; Hirama, M.
Angew. Chem. Int. Ed. 2006, 45, 4843–4848.
1. Total Synthesis of Merrilactone A
*Inoue, M.; Sato, T.; Hirama, M.
J. Am. Chem. Soc. 2003, 125, 10772–10773.
Review, Account
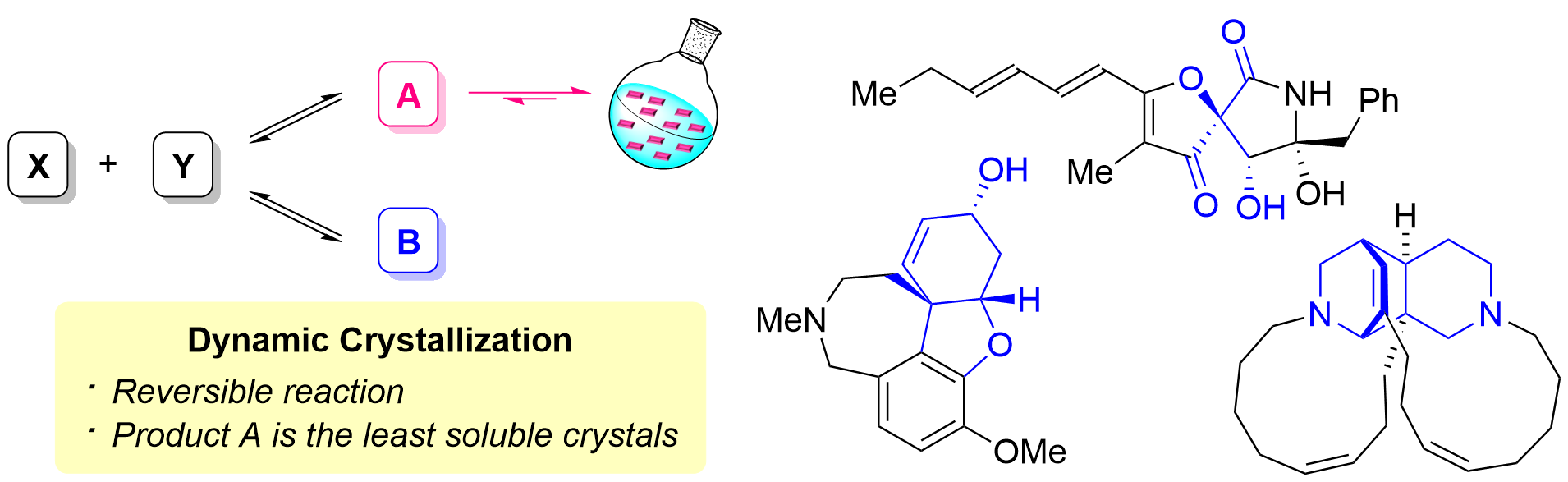
8. Synthesis of Natural Products and their Derivatives Using Dynamic Crystallization
*Takaaki Sato
Chem. Lett. 2025, 54, upae244.
This is an invited review.
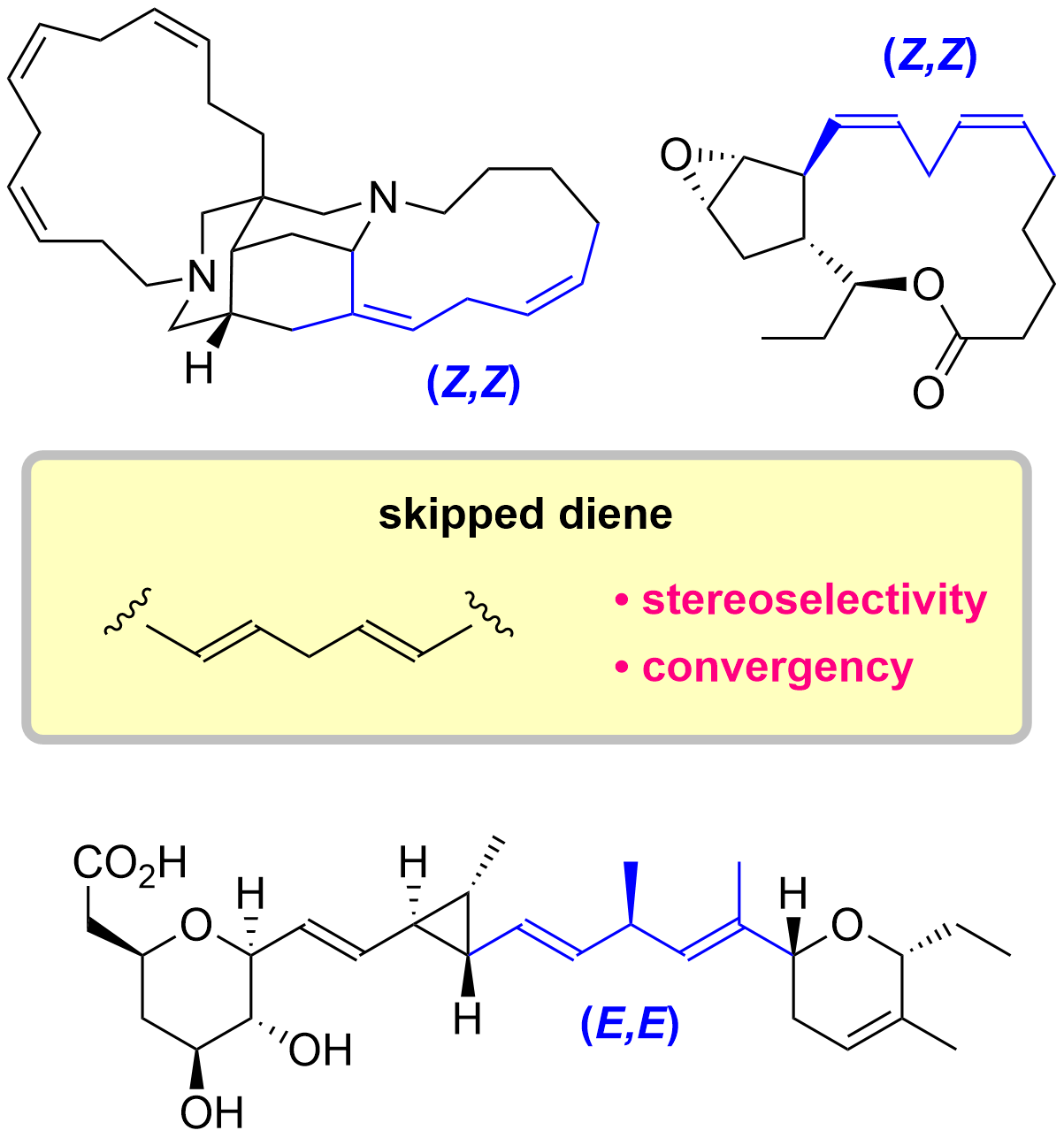
6. Total Synthesis of Skipped Diene Natural Products
*Sato, T.; Suto, T. Nagashima, Y.; Mukai S. Chida, N.
Asian J. Org. Chem. 2021, 10, 2486–2502.
This is an invited review.
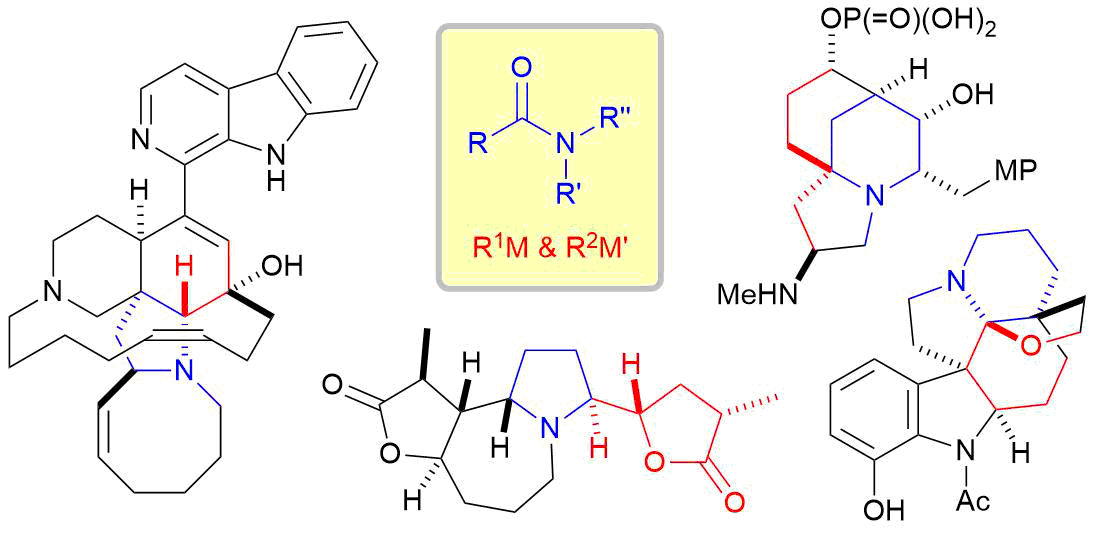
5. Total Synthesis of Complex Alkaloids by Nucleophilic Addition to Amides
*Sato, T.; Yoritate, M.; Tajima, H.; Chida, N.
Org. Biomol. Chem. 2018, 16, 3864–3875.
This is an invited review.
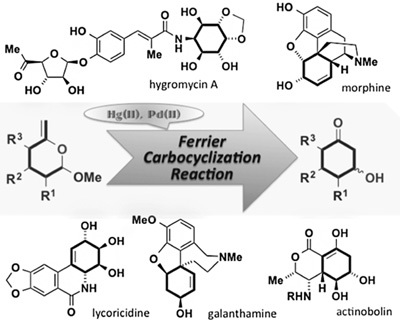
3. Synthesis of Natural Products Containing Cyclohexane Units Utilizing the Ferrier Carbocyclization Reaction
*Chida, N.; Sato, T.
Chem. Rec. 2014, 14, 592–605.
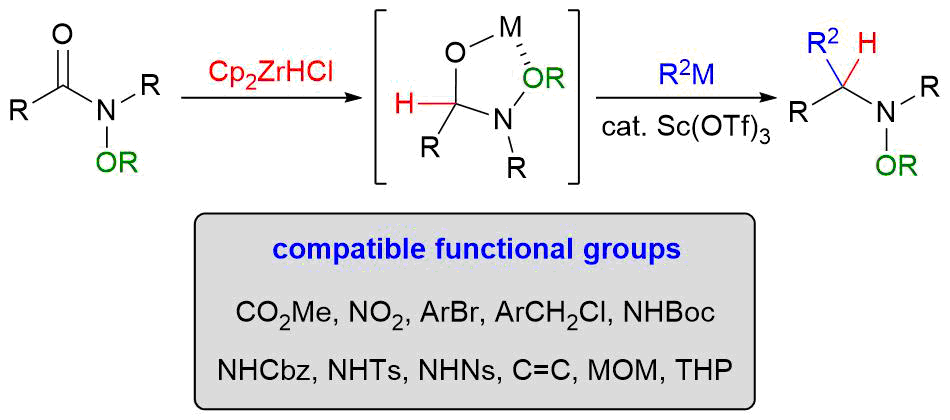
2. Nucleophilic Addition to N-Alkoxyamides
*Sato, T.; Chida, N.
Org. Biomol. Chem. 2014, 12, 3147–3150.
This is an invited account.
This article was selected as one of the highly cited articles.
1. メリラクトンAの全合成:遠隔不斉誘導と不斉非対称化
井上将行, 佐藤隆章, 平間正博
有機合成化学協会誌 2007, 65, 419–429.
Book
6. Unified Total Synthesis of Madangamine Alkaloids
5. 博士研究員からはじまるアミド基への求核付加反応
佐藤隆章
ドラマチック有機合成化学 感動の瞬間100, 有機合成化学協会編, 化学同人, 2023, pp. 142‒143.
4. Nucleophilic Addition to Amides Toward Efficient Total Synthesis of Complex Alkaloids
Sato, T.
New Tide of Natural Product Chemistry, Ishikawa, H.; Takayama, H.; Springer, 2023, pp. 275‒293.
3. Chiral Pool Synthesis: Chiral Pool Syntheses Starting from Carbohydrates
Chida, N.; Sato, T.
Comprehensive Chirality, Carreira, E. M.; Yamamoto, H.; Elsevier Science, 2012, pp. 207‒239.
2. [2+2]光付加環化
井上将行, 佐藤隆章
天然物合成で活躍した反応 実験のコツとポイント, 有機合成化学協会編, 化学同人, 2011, pp. 62‒63.
1. 対称性を利用したメリラクトンAの全合成 遠隔不斉誘導と不斉非対称化
井上将行, 佐藤隆章, 平間正博
天然物全合成の最新動向, 監修 北泰行, シーエムシー出版, 2009, pp. 3‒17.
Others
5. アミド基選択的な求核付加反応の開発と応用
佐藤隆章
化学と工業, 2017, 70, 621–622.
4. アミド基選択的な求核付加反応による天然物全合成の効率化
佐藤隆章
化学と工業, 2014, 67, 786.
3. Cover Profile: Two-step Synthesis of Multi-substituted Amines Using N-Methoxy Group as a Reactivity Control Element
Yoritate, M.; Meguro, T.; Matsuo, N.; Shirokane, K.; *Sato, T.; *Chida, N.
Chem. Eur. J. 2014, 20, 7849.
2. カチオン-π相互作用を利用した有機合成化学
千田憲孝、佐藤隆章
化学, 2008, 63, 66-67.
1. 酸化的カップリング反応を用いたインドールアルカロイド類の全合成
佐藤隆章
有機合成化学協会誌, 2006, 64, 679–670.