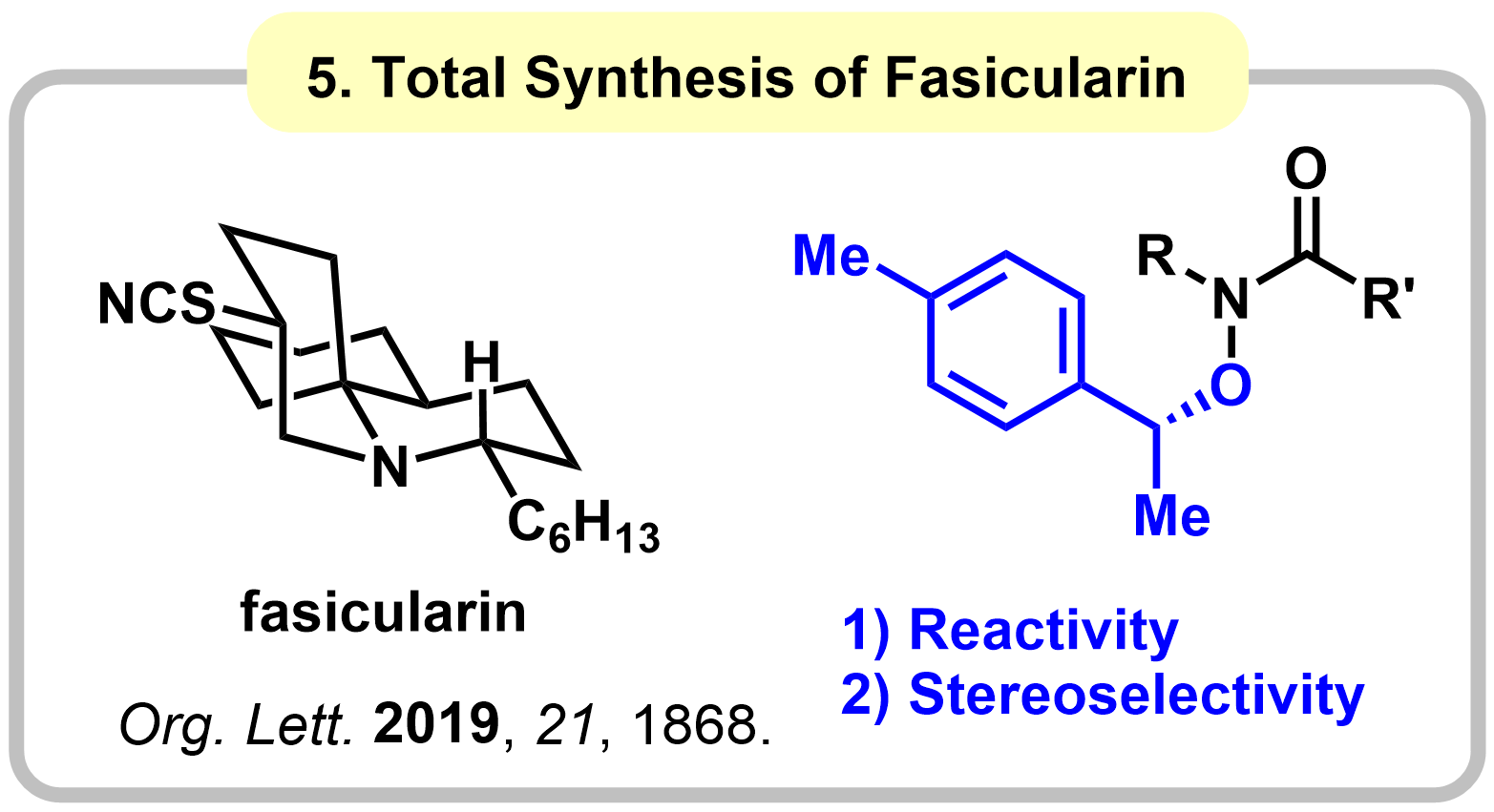
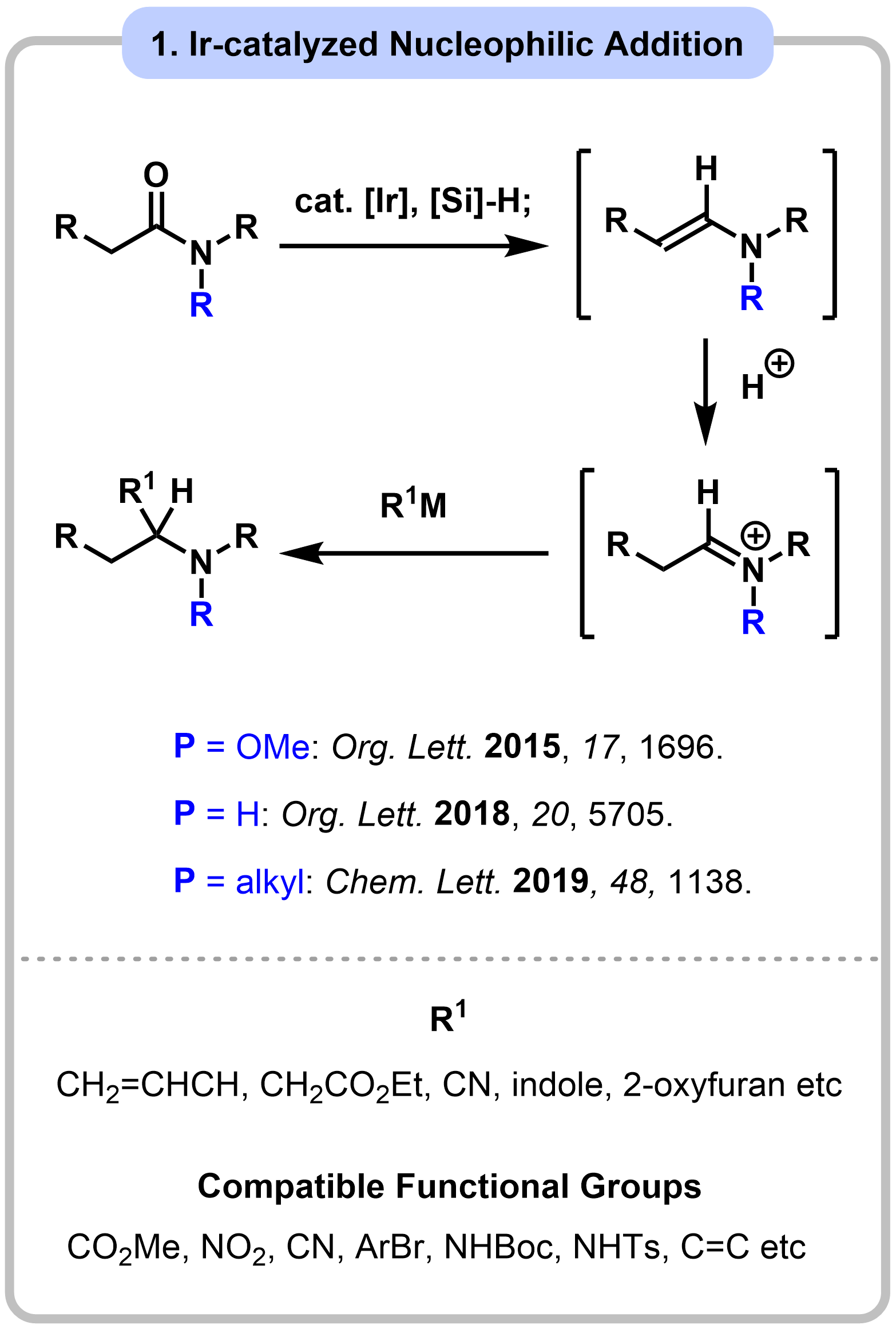
Research 1. Functionalization of Amides: Development and Application to Total Synthesis
Amide functional groups play a significant role in a variety of areas such as medicinal drugs. Therefore, the efficient synthesis of amide bonds has received much attention by organic chemists, making this one of the most reliable reactions in synthetic organic chemistry. On the other hand, functionalization of the generated amide groups is less explored than their construction due to the high stability of amide carbonyls. Our group has been working on development of practical transformations of amides, and application to total synthesis of complex natural products.
1-1. Reactivity Control of Amides Using Alkoxy Group
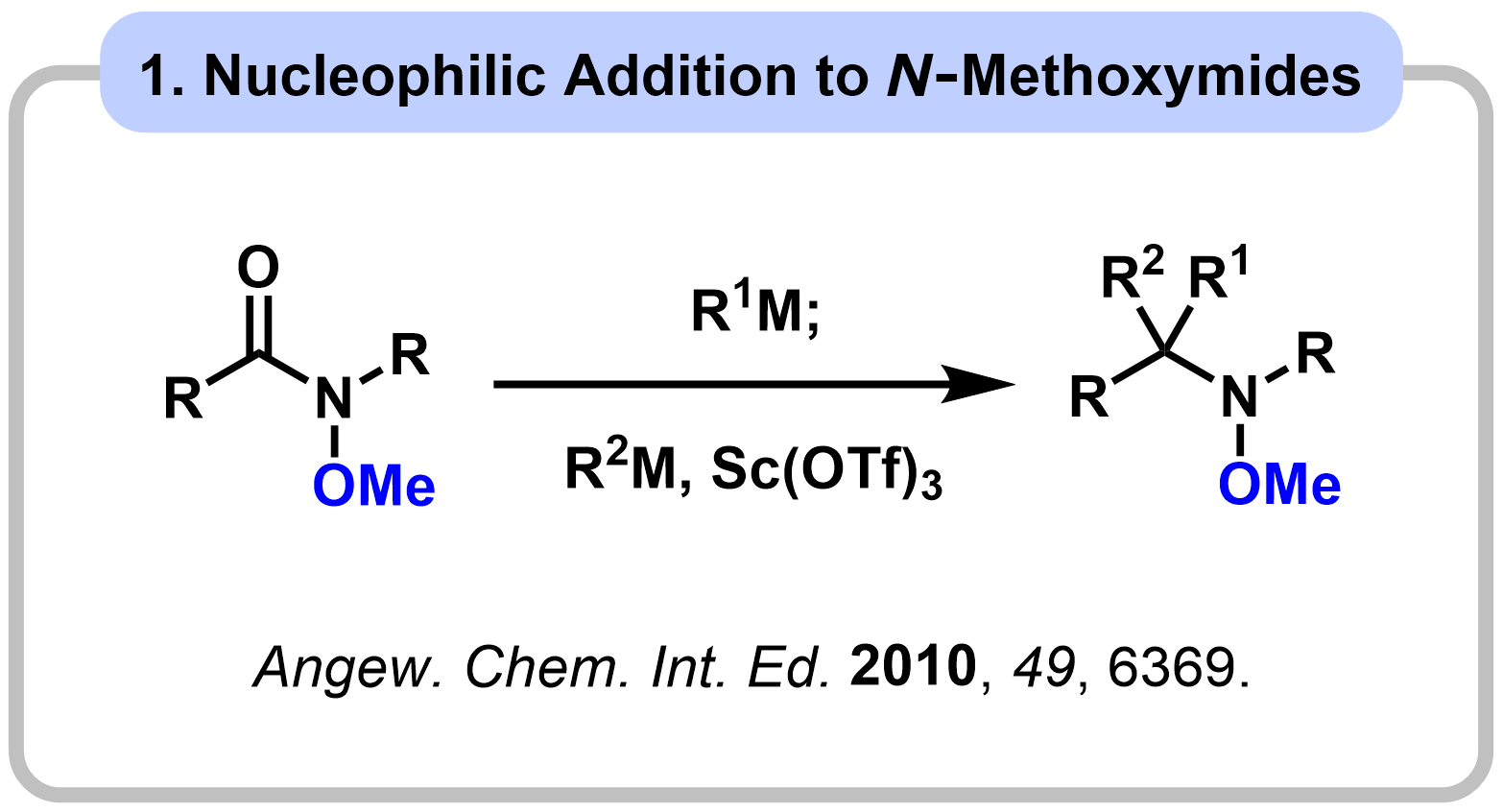
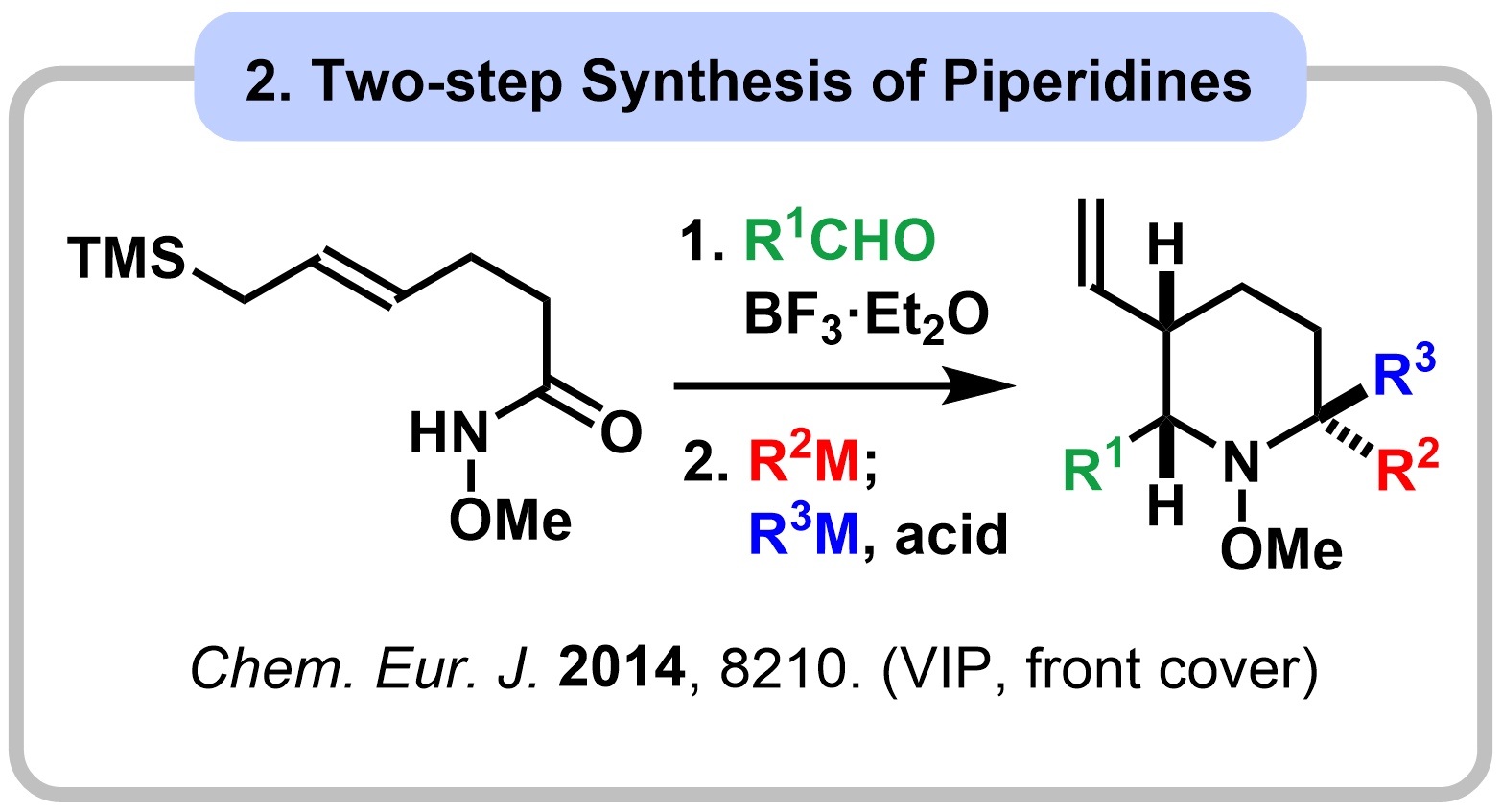
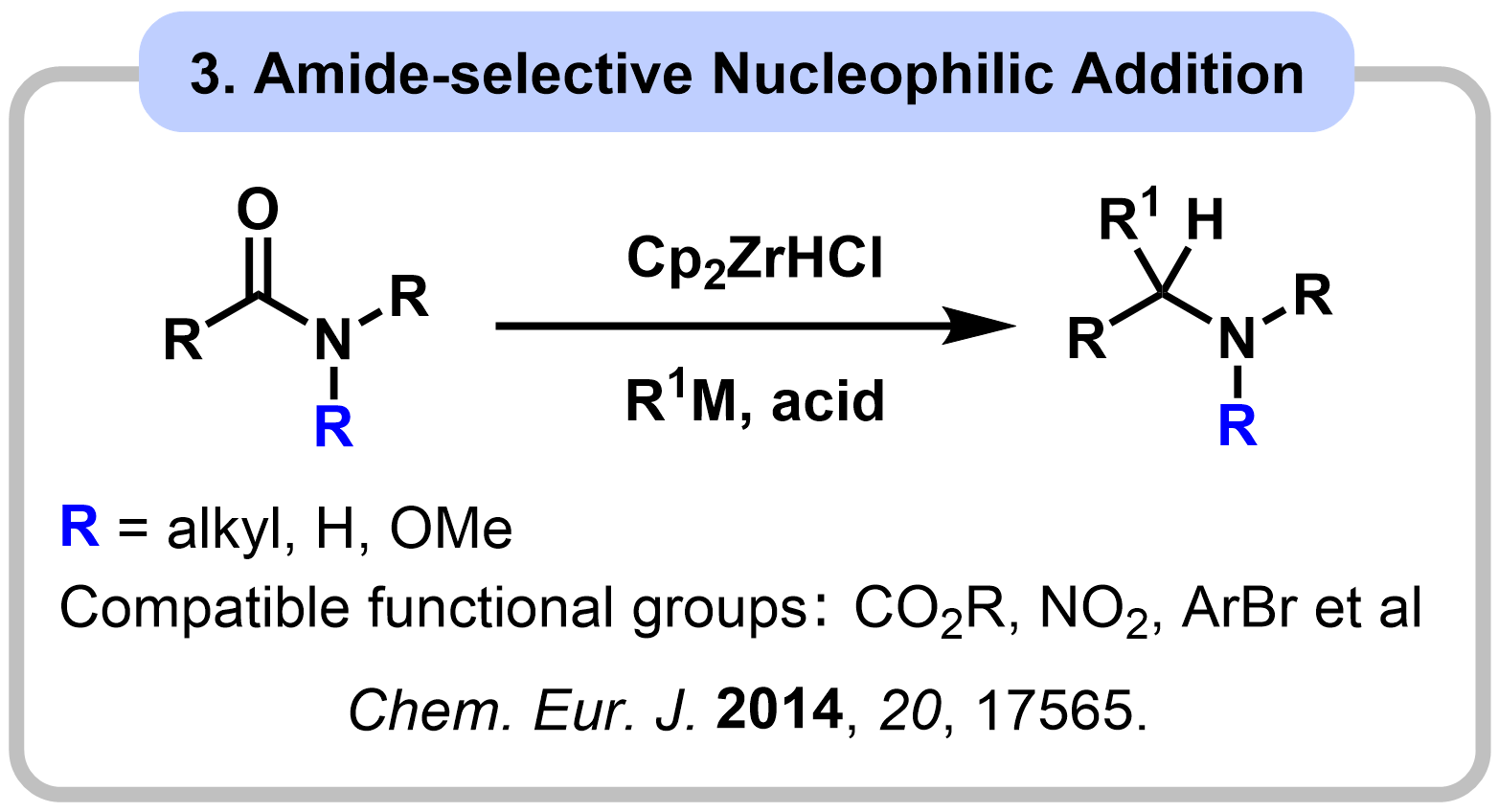
|
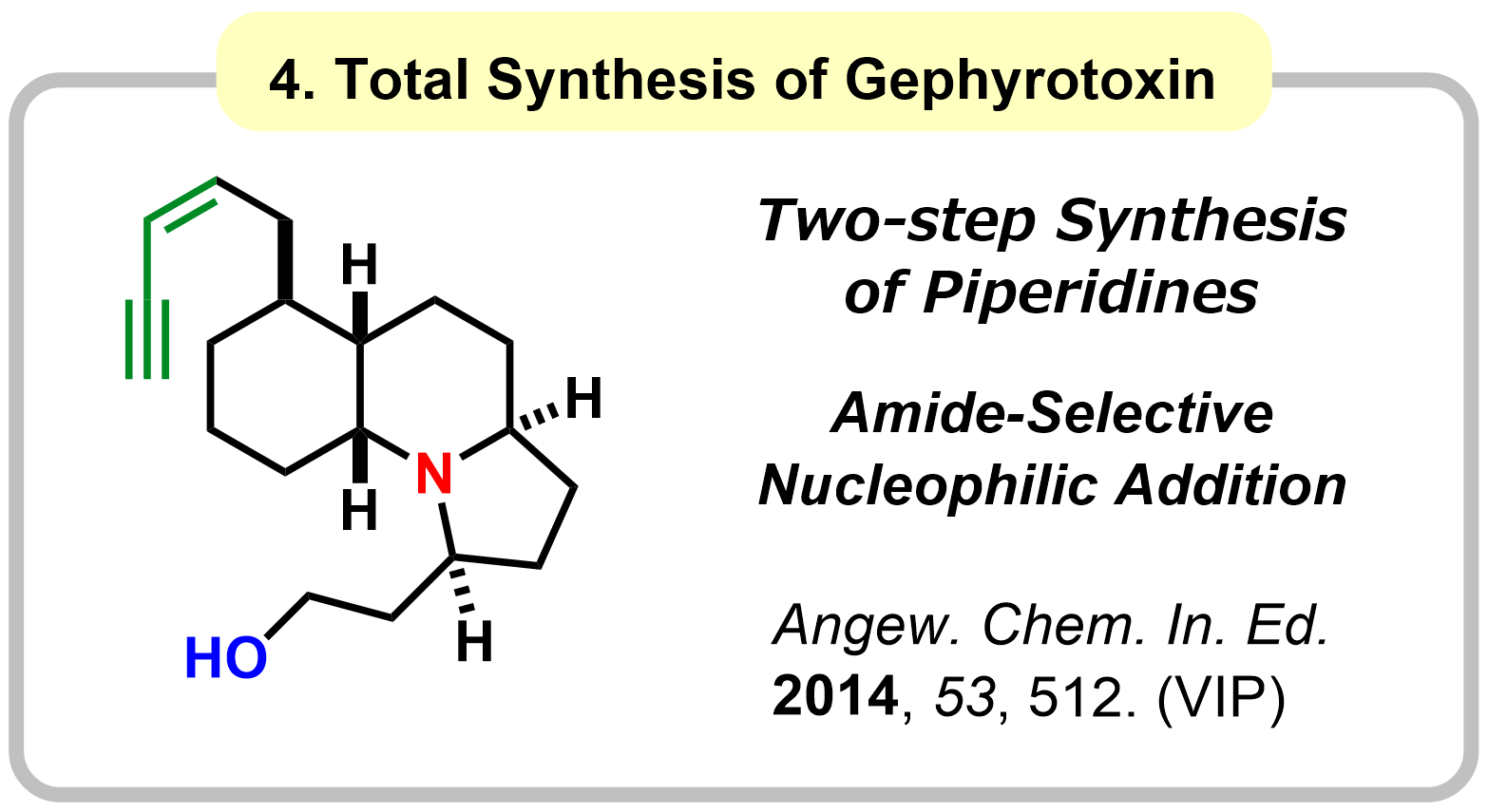
|
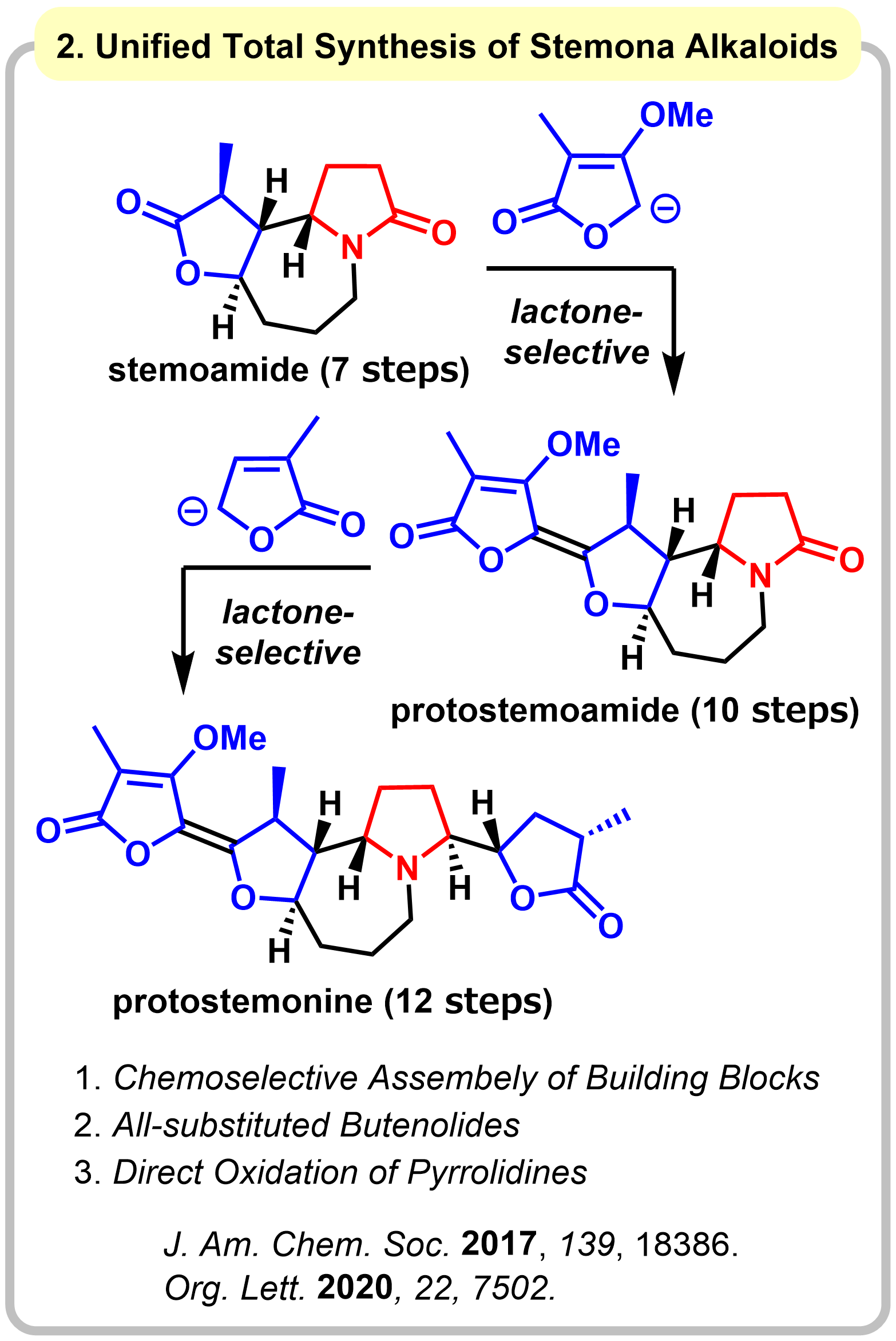
1-2. Unified Total Synthesis of Stemoamide-type Alkaloids
|
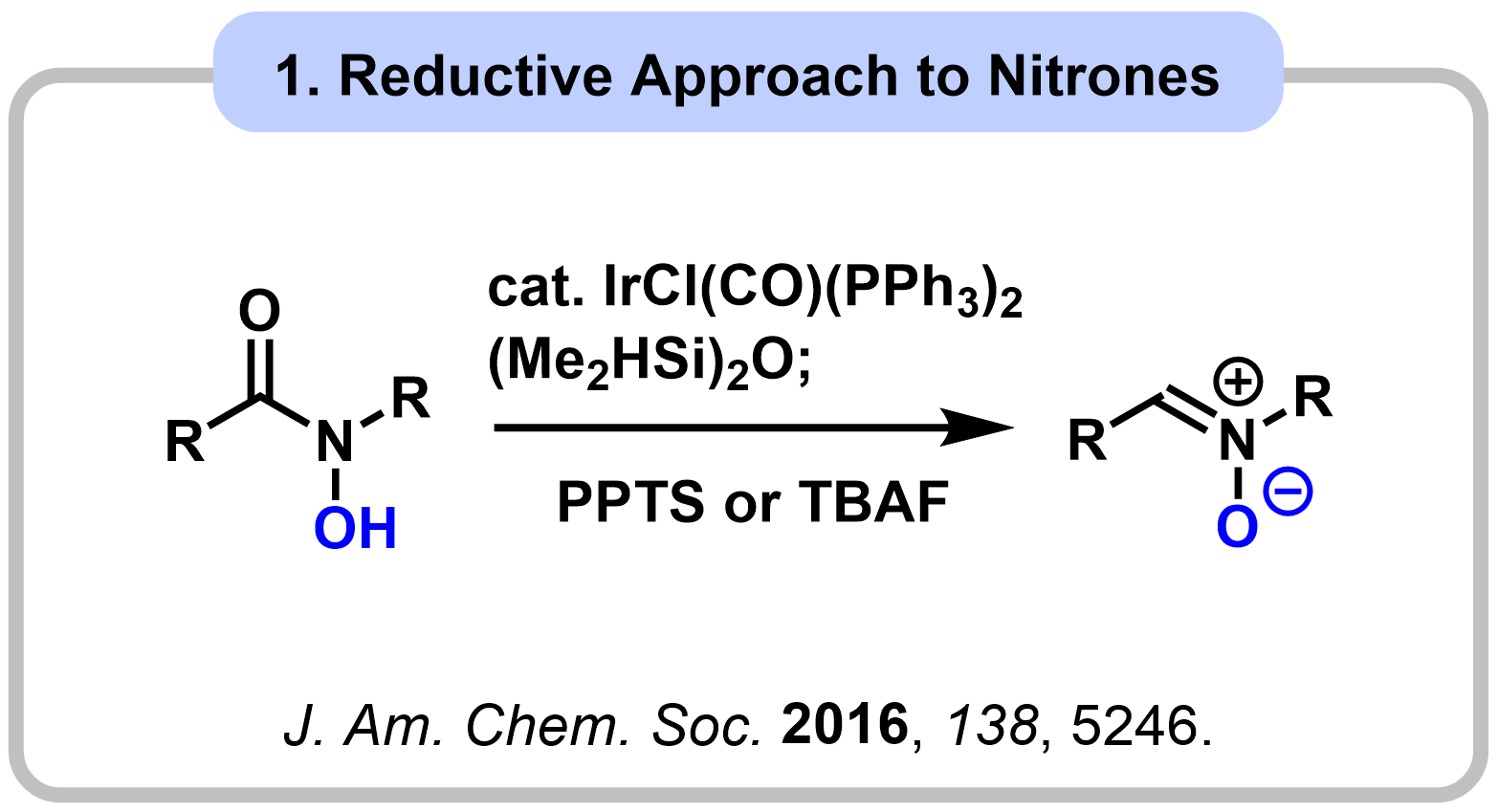
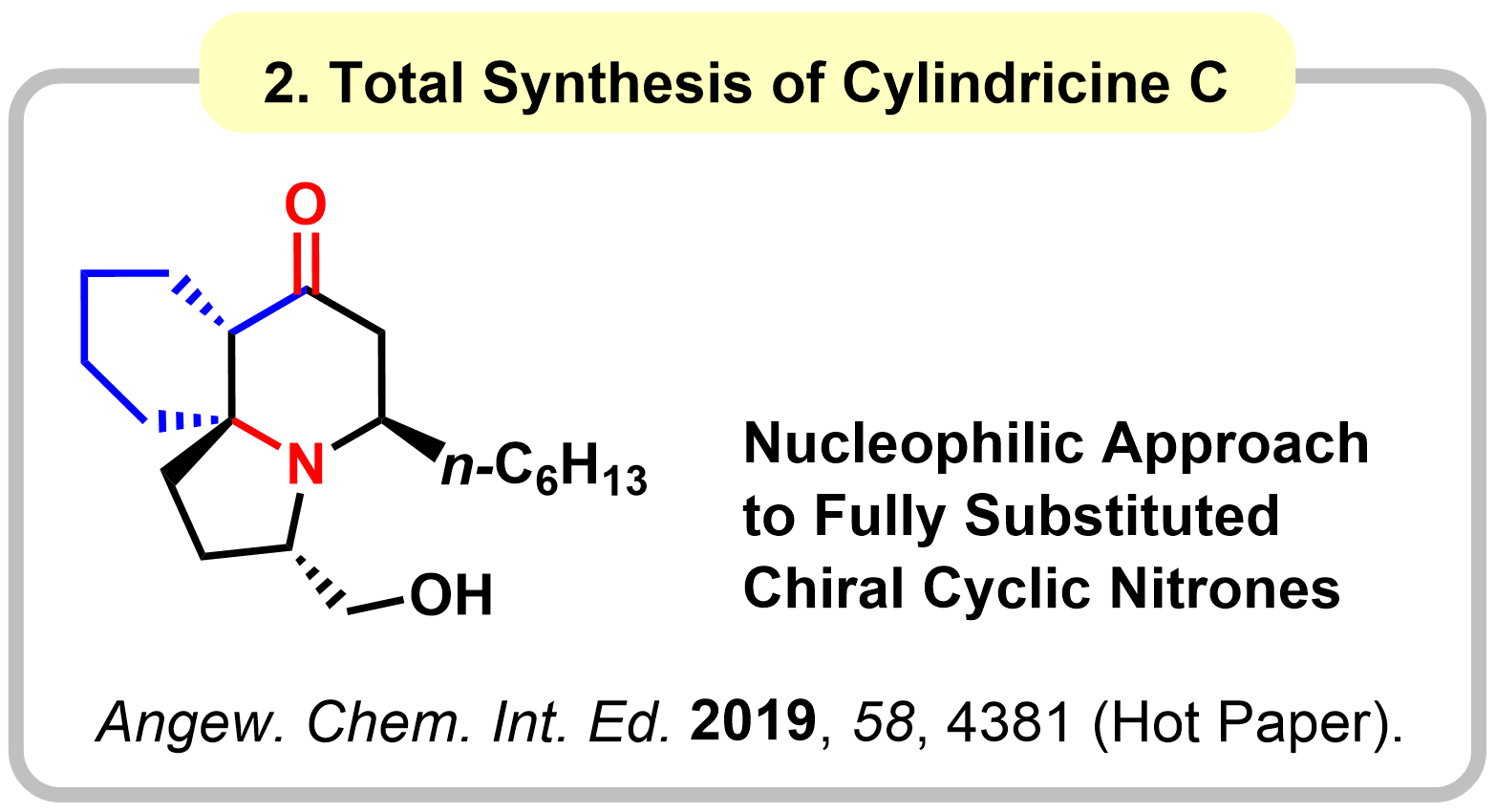
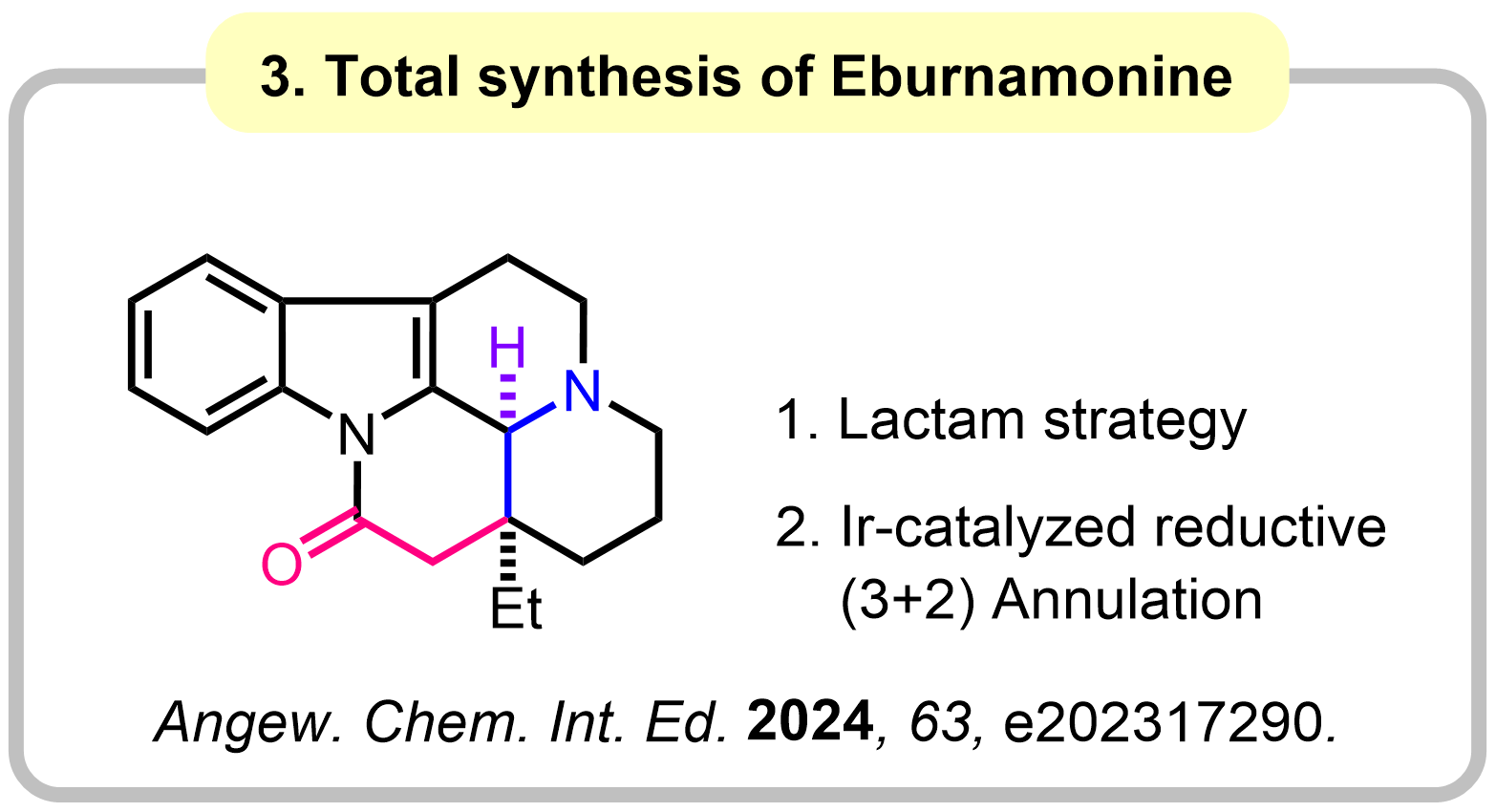
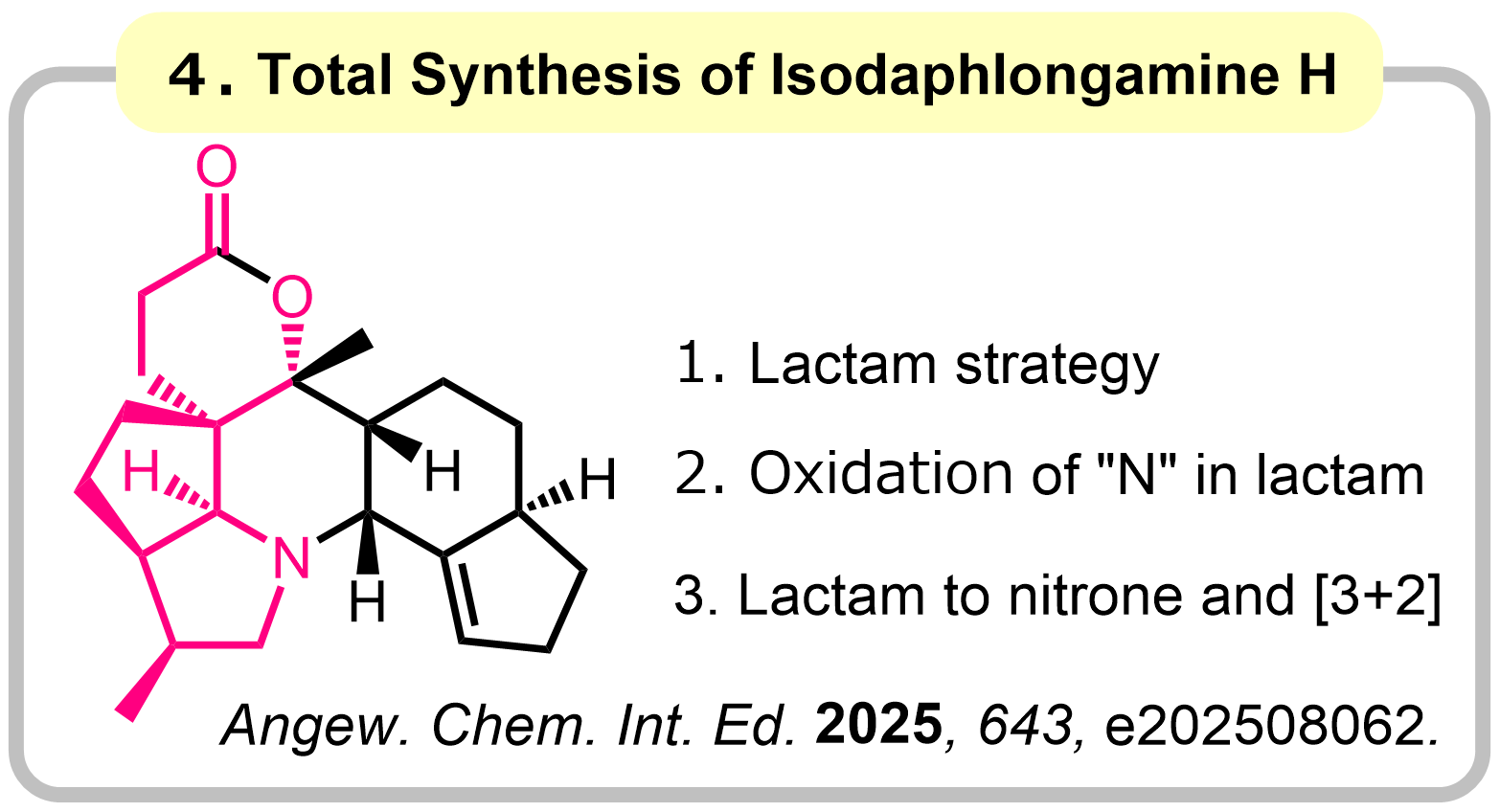
1-3. Amides as 1,3-Dipole Equivalents
|
|
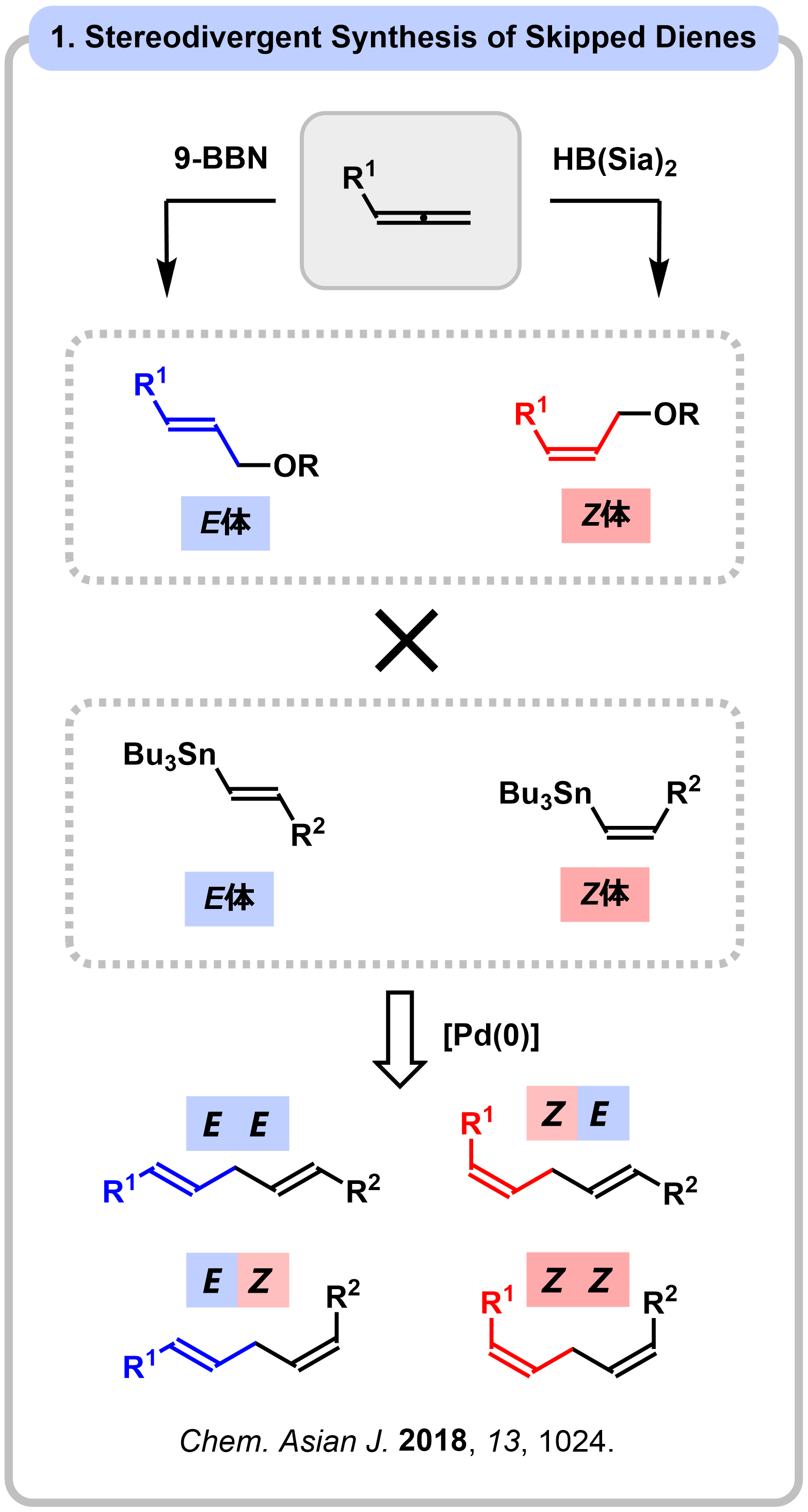
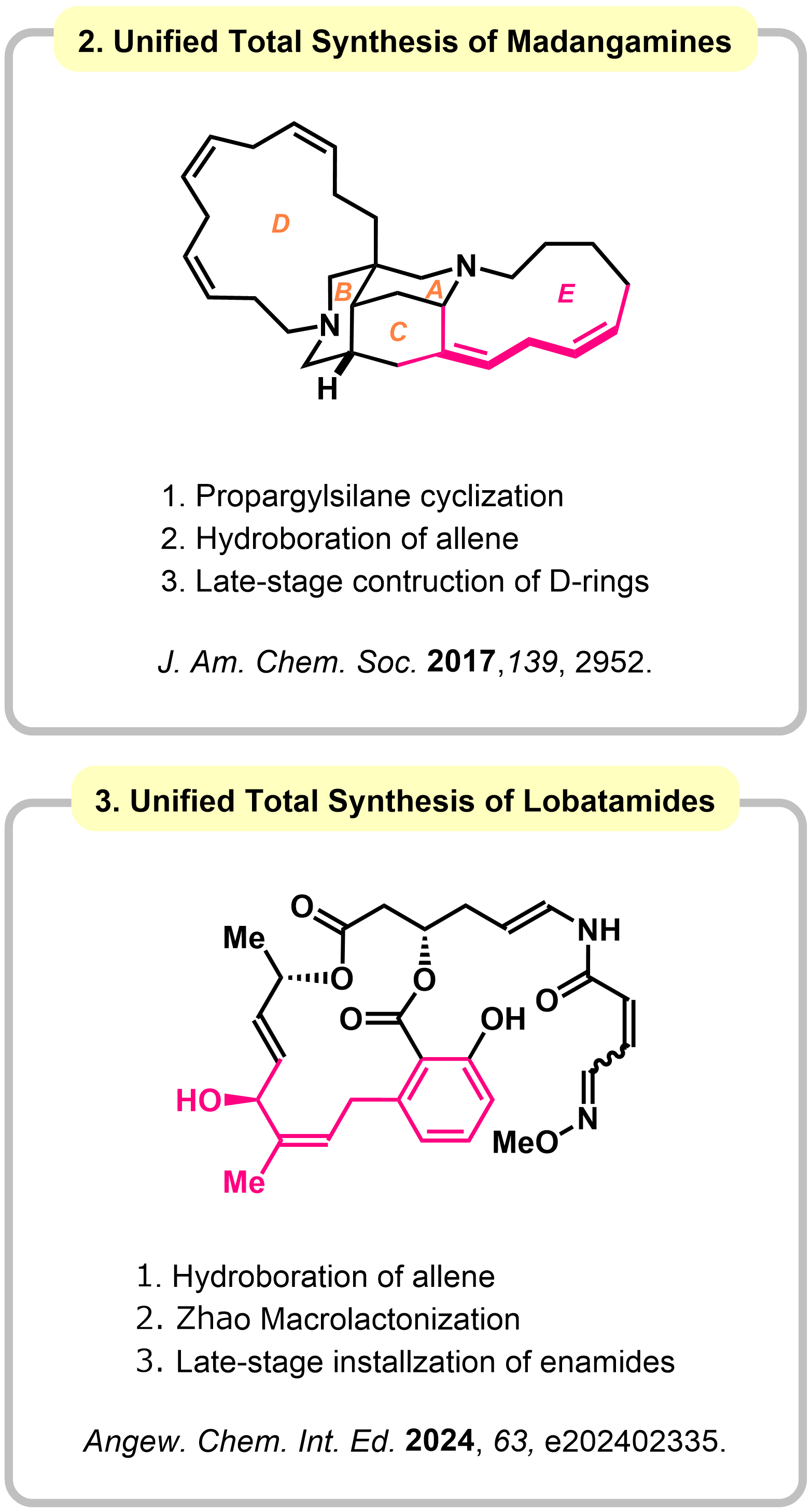
Research 2. Synthesis of Natural Products through Hydroboration of Allenes
An allene is a unique functional group containing two contiguous carbon@carbon double bonds. Transformation of Allenes have been expected as an attractive intermediate in the synthesis of complex molecules, although control of multi-selectivity is not trivial due to the distinct reactivities derived from the two orthogonal p-bonds. We have developed stereodivergent hydroboration-oxidation by changing the substituents of boranes. The method was successfully applied to the unified total synthesis of madangamines and lobatamides.
|
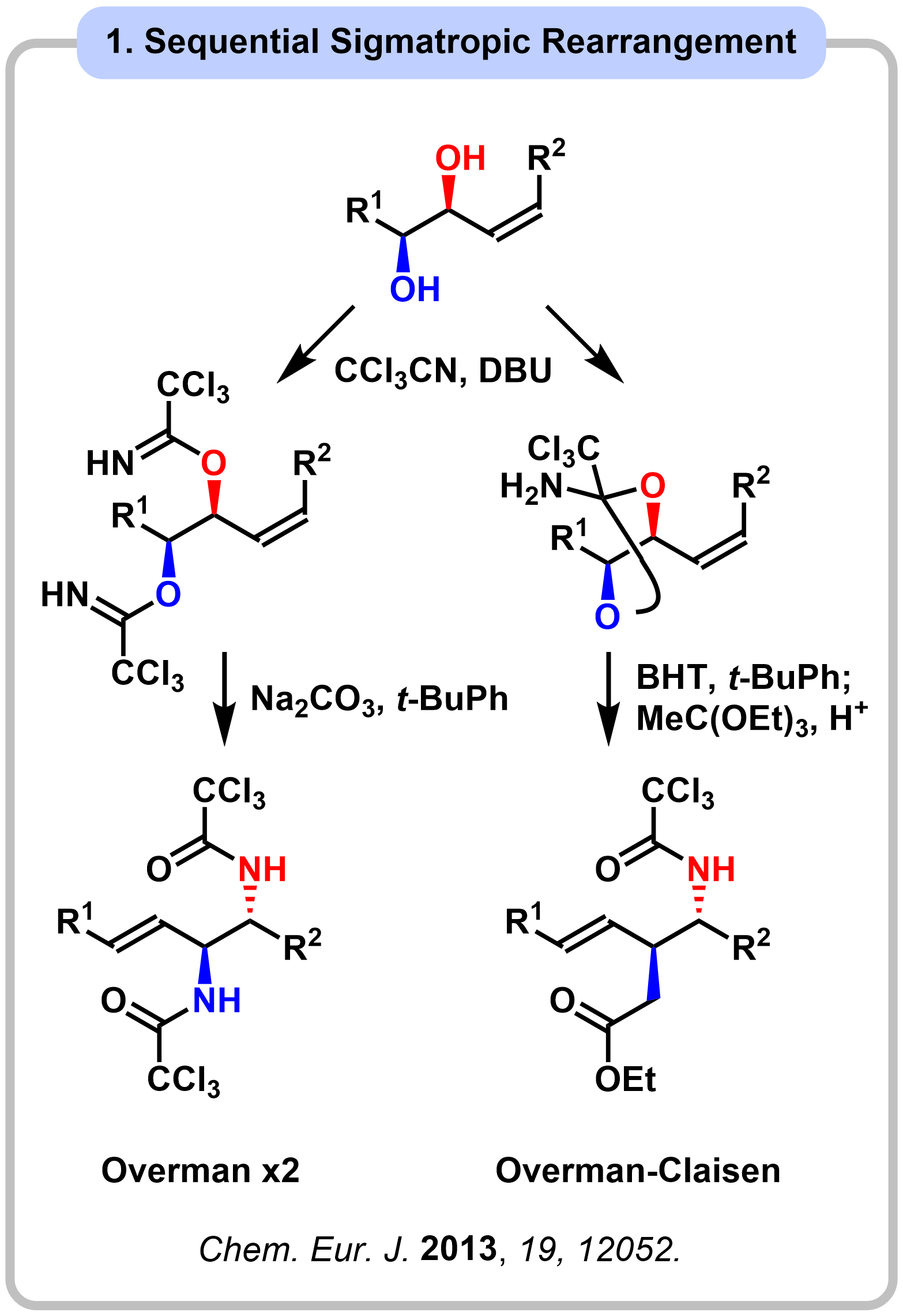
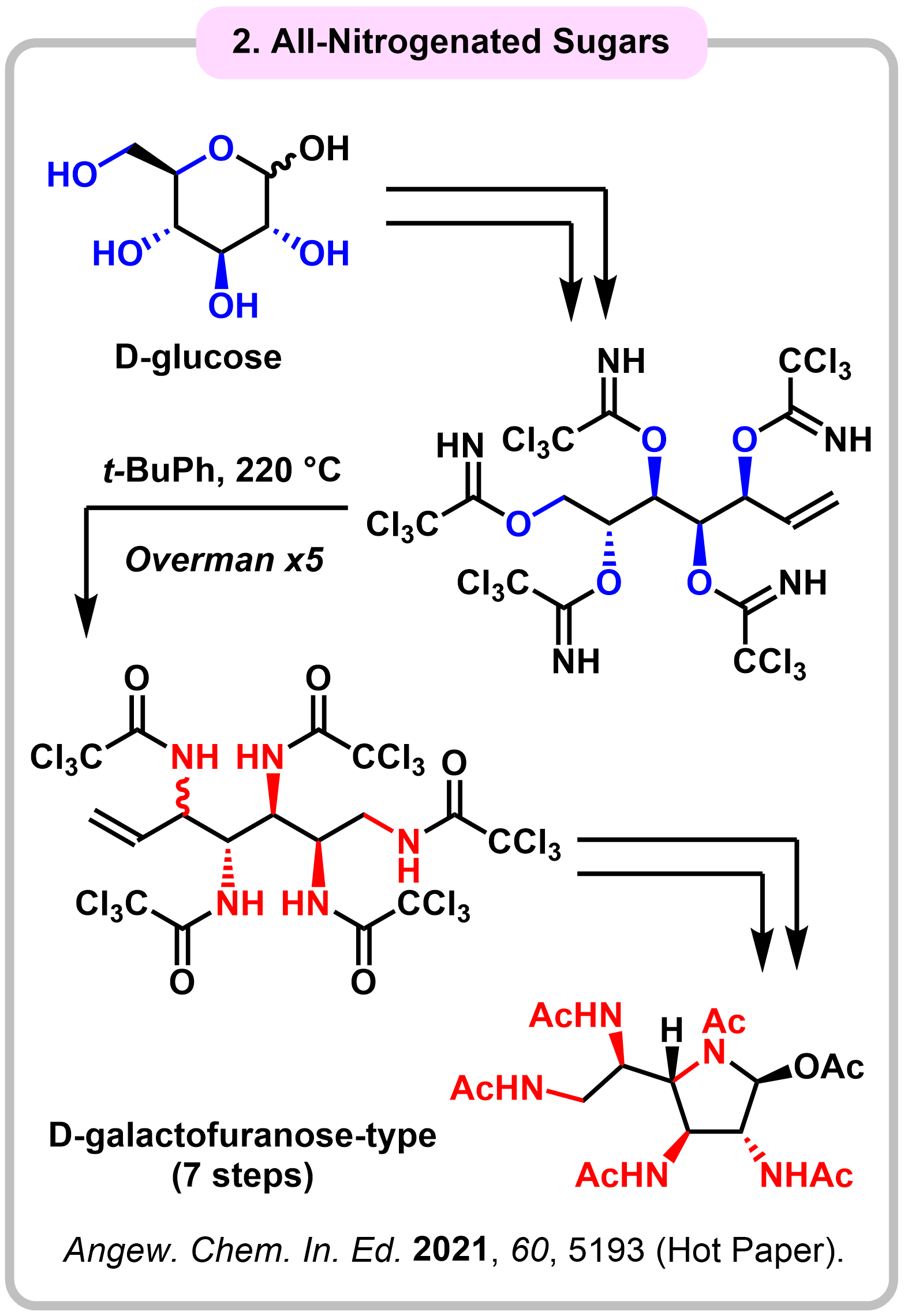
Research 3. Synthesis and Evaluation of All-Nitorgenated Sugars (ANSs)
All-nitrogenated sugars (ANSs) are anticipated to be privileged structures with useful biological activities. However, ANS synthesis has been challenging due to the difficulty in the installation of multi-amino groups. We developed a concise synthetic route to peracetylated ANSs in seven steps from commercially available monosaccharides. The key to success is the use of the sequential Overman rearrangement, which enables formal simultaneous substitution of four or five hydroxy groups in monosaccharides with amino groups.
|